Advanced imaging shows the mouse heart as it pumps, leading to insights into cardiac physiology and disease genesis.
ANNE BUGLIONE AND NOZOMI NISHIMURA, CORNELL UNIVERSITY
Most people know the heart pumps blood to the body, but they may not realize that the blood that fills the four chambers of the heart provides no sustenance to the myocardium, or the heart muscle, itself. Instead, a separate blood supply, the coronary circulation, runs along the surface of the heart, penetrates the tissue, and branches into a network of capillaries. Blockages that decrease blood flow in the coronary circulation are the cause of heart attacks, which affect one American approximately every 39 s1. Although the coronary arteries have been well studied, blood flow through the microscopic myocardial capillaries remains poorly understood. Fortunately, new techniques in multiphoton microscopy are helping to change this.
Courtesy of iStock.com/Ugreen.
Many lower-resolution imaging modalities are available for investigating the heart, including magnetic resonance imaging, computed tomography, and ultrasound. These technologies are invaluable for use in the clinic for performing diagnoses and interventional procedures, and they are used in research in both patients and animal models for investigation of disease. Some of these imaging modalities can measure rapid motion and provide an assay of heart function, which is critical for many diagnoses. However, they are limited to showing relatively large structures.
Studying capillary blood flow and other cellular processes in the heart at a microscopic level has been exceptionally challenging, primarily because the heart is constantly moving. Consequently, much remains unknown about cardiac physiology. Better understanding of cardiac physiology is both of great interest to the scientific community and critical for developing novel treatments for cardiovascular disease, a leading cause of mortality worldwide. Recently, developments in multiphoton microscopy for rodent models enabled visualization at the cellular scale within the beating heart, finally allowing researchers to answer questions about cell behavior and physiology within the context of the heart’s motion.
Multiphoton cardiac imaging
Multiphoton microscopy, which includes two-photon microscopy, enables visualization of the action of cells within living animals. Coupling the method with novel functional markers, such as calcium indicators that light up when cells are active, allows in vivo imaging of cellular behaviors such as the firing of neurons and the flow of blood. Multiphoton microscopy has been used for the examination of many organ systems — such as the brain, skin, and gastrointestinal tract — and its use is increasingly common in fields such as neurobiology, cancer research, and immunology. Its application in cardiac research has lagged because of the challenges in imaging a moving organ located deep within the chest. Recently, advancements in surgical approaches and instrumentation have extended multiphoton microscopy to the mouse heart.
Multiphoton microscopy is a variation of fluorescence microscopy that uses nonlinear excitation to limit fluorescence excitation to a single point at the focus of a high-intensity laser. In two-photon microscopy, two photons must interact nearly simultaneously (within 10−16 s) with a fluorescent molecule to excite fluorescence, so that the emitted fluorescence is proportional to the square of the excitation intensity. In three-photon microscopy, three photons must interact, and the signal is proportional to the excitation intensity cubed. Compared to two-photon microscopy, three-photon microscopy uses longer wavelengths, which can penetrate deeper into tissues and allow imaging at greater depths. In both two- and three-photon microscopy, the signal is primarily produced at the beam focus, where excitation intensity is highest.
An image is produced by scanning the focus in the sample, measuring the emitted fluorescence, and assigning the value to the image pixel corresponding to the position. Because fluorescence is produced at only one point at a time, scattered signal photons can also be detected and assigned to their point of origin, so that the imaging is relatively insensitive to scattering in tissues. Overall, this means that the signal comes from only a small volume, and a three-dimensional image volume is reconstructed with limited background from the out-of-focus areas.
Multiphoton microscopy takes advantage of strategies that use fluorescence to label specific structures or cell types. Fluorescent labels include dyes — such as intravenous dyes injected into the animal’s bloodstream at the time of imaging — or proteins genetically encoded in transgenic animals. For example, mouse lines can be bred to have fluorescent reporters in multiple cell types of interest, enabling the specific cells to stand out during imaging. The ability to image cell dynamics with micrometer resolution in intact tissues and living animals provides unique information about health and disease that cannot be obtained with other imaging modalities.
Imaging the beating heart
Earlier attempts at cardiac imaging used preparations that took the heart out of its natural position, limiting the utility of the data collected. In the Langendorff perfusion method, the heart is removed from the chest and perfused with a solution similar in composition to that of blood. This allows easy manipulation and stabilization and has provided valuable initial insights into cardiac physiology. However, because the heart is removed from the systemic circulation and lacks feedback from the brain and other systems in the body, the relevance of the findings is limited. In the heterotopic explantation method, a donor heart is surgically removed and relocated to a recipient abdomen or neck2. Although this method enables studies of transplantation and host-graft interactions, the environment and circulation of the heart are perturbed. Imaging the heart in the natural position in the chest is necessary to fully capture cellular interactions and activity under normal physiological conditions.
Recent advancements in surgical approaches3-5 have enabled the use of multiphoton microscopy in the actively beating heart in rodents (Figure 1a). To image the left ventricle, the largest chamber of the heart, which pumps blood to the systemic circulation, the mouse is anesthetized and positioned left side up. An incision is made through the chest wall, between the ribs, to access the heart. Throughout surgery and imaging, vital functions are monitored and maintained. A small animal ventilator breathes for the mouse and maintains inflation of the lungs after the chest is opened. The heart rate and rhythm are recorded with an electrocardiogram (ECG), which helps to ensure that the heart is beating normally and can sustain the animal throughout the surgery and imaging. Signals from the ventilator and ECG are recorded and used later during image processing.
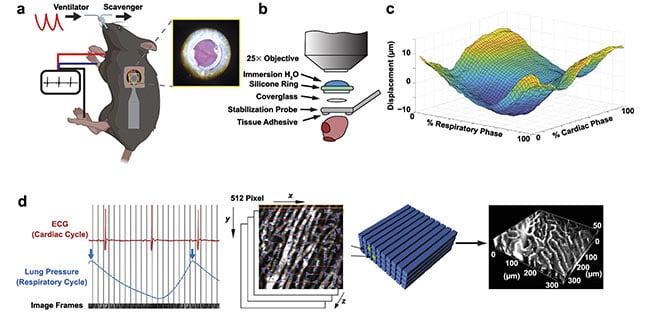
Figure 1. The surgical approach for intravital cardiac imaging in a mechanically ventilated mouse (a). The heart surface showing coronary vessels (a, inset) stabilized by a glass window (b). Residual motion from the heartbeat and respiration (c). For reconstruction of images, rows of image voxels are assembled into an image volume associated with a particular combination of cardiac and breathing phase (d). Adapted with permission from Reference 7.
A variety of methods have been developed to minimize motion artifact from breathing and heartbeat to enable intravital imaging of various organs. For example, to image the brain, the head is held in place by stabilizing the rigid skull. Many organs are lightly compressed with sufficient pressure to reduce motion without interfering with normal physiology. Allowing the natural motion of the heart to continue is essential to keep the animal alive and to ensure that data is reflective of natural conditions. At the same time, motion in the imaging region needs to be minimized.
To accomplish this, a rigid 3D-printed titanium probe (Figure 1b), which supports the imaging window, is adhered to the surface of the heart. Blood continues to flow under the window (Figure 1a). Alternative methods for stabilization include endoscopic suction, or negative pressure, using a device similar to the surgical stabilizers used in human heart surgery. This type of stabilizer can be removed and reattached to the heart to image multiple regions in one animal. The stabilizer is also very small, so it can access the heart through a relatively small incision.
Heartbeat dynamics
Stabilization methods greatly reduce the motion of the heart but do not eliminate it completely. The position and shape of the heart still change as the heart beats and as the lungs inflate during respiration (Figure 1c). Image reconstruction in post-processing can improve visualization and enable quantitative analysis. During imaging, high-speed scanning at 30 fps (8-kHz line rate) enables sufficient real-time visualization to navigate to the region of interest. The electrical signal from the ECG and the lung pressure from the ventilator are recorded to document the timing of heartbeats and respirations. Image segments are indexed according to when they occurred in the cardiac cycle (defined by the electrical peaks, such as R waves, in ECG) and the phase in the respiratory cycle (defined by the lung pressure). Image volumes are reconstructed by assembling image segments from specified ranges in the 2D phase space, so that measurements are made as a function of both the cardiac and breathing cycles (Figure 1d).
Another way to minimize motion is to image only during part of the cardiac cycle, such as when the heart is relaxed. This approach is commonly used in medical imaging. Alternatively, the mouse’s heart can be electrically paced rather than allowing spontaneous contraction. Image acquisition can then be triggered to occur at specific parts of the cardiac cycle. However, information about the other parts of the cycle is not captured. Similarly, the ventilator can be briefly paused to eliminate motion due to respiration. However, this removes the impact of breathing on measurements and cannot be used in models that are sensitive to brief oxygen deprivation.
Diseased and healthy hearts
Multiphoton microscopy can be used to study many aspects of physiology in both healthy hearts and disease models. Even capillaries, the smallest and most numerous vessels, are visible in two-photon microscopy (Figure 2a). Intravital microscopy provides a unique view of the tiny structures and how they are affected by heart motion. Red blood cells can be seen moving through the capillary vessels in single file (Figure 2b). White blood cells, such as neutrophils, can be labeled with proteins (antibodies) that bind to structures on the surface of specific cells (Figure 2c, left), or by expression of fluorescent proteins in transgenic animals (Figure 2c, right).
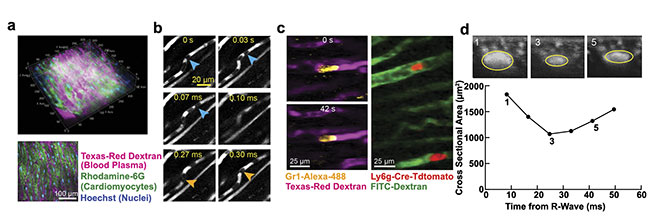
Figure 2. Two-photon microscopy reveals cells and individual capillaries within the ventricular wall in a mouse heart (a). Individual red blood cells (blue and orange arrows) (b) and neutrophils (c, left) moving within capillaries (c, right). Cross-sectional views of a blood vessel show compression throughout the cardiac cycle (d). Courtesy of Cornell University.
Capillaries are arranged in a branching network (Figure 2a) and form the connection between the larger vessels (arteries) that supply and return blood (veins) from the myocardium. Capillaries are the site of nutrient exchange and run between cardiac muscle cells. Because of this arrangement, flow in myocardial vessels is subject to pressure variations throughout the cardiac cycle. This variation in pressure causes a measurable change in vessel diameter in images reconstructed throughout the cardiac cycle (Figure 2d). Cyclic decreases in diameter lead to corresponding increases in vessel resistance; this is a normal physiologic phenomenon.
However, disease can also increase resistance to blood flow, by reducing vessel diameter or by other means, such as slowed movement of cells in capillaries. In some cases, white blood cells become transiently stalled or “stuck” in capillary segments, which prevents forward flow of red blood cells, similar to a traffic jam. In heart attacks, such blockages are thought to contribute to persistent blood flow deficits, which remain even after blockages in larger vessels are removed. Little is known about this “no-reflow” phenomenon because of the difficulty in studying microscopic flow in the beating heart.
In addition to identifying cellular motion, multiphoton microscopy can measure cellular activity by using functional indicators. When heart muscle cells are activated and they contract, intracellular calcium concentration increases. This activation is detectable using genetically encoded proteins such as GCaMP, which increase fluorescence intensity as the calcium concentration increases. Changes in calcium in cardiac muscle cells over the course of the cardiac cycle can be measured in individual cells (Figure 3a). Imaging in live mice enables the study of the relationships between action potentials, contractility, and local blood flow. GCaMP can also be used to study the physiology of other types of cells, including endothelial cells, which line the capillaries (Figure 3b). In vivo microscopy is also compatible with the optical control of cell activation using actuators such channelrhodopsin (Figure 3c)4,6.
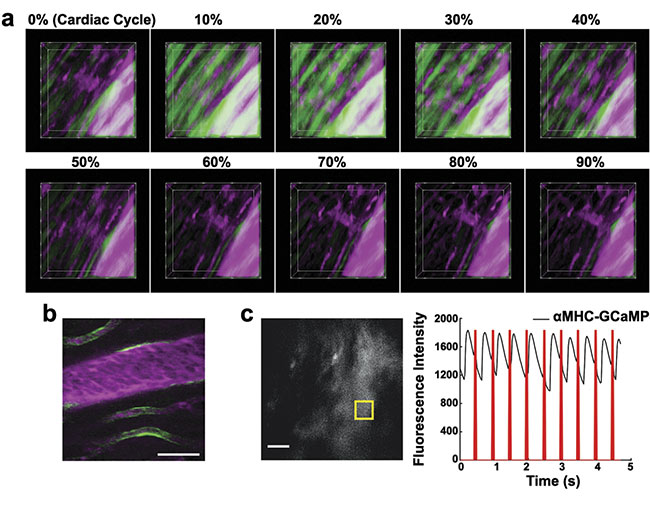
Figure 3. Genetically encoded proteins provide additional functional markers. Action potentials increase fluorescence of GCaMP in cardiomyocytes (a). GCaMP in endothelial cells (b). Optogenetic cardiac pacing by optical excitation of channelrhodopsin results in wide-field fluorescence changes in a mouse that expressed GCaMP in cardiomyocytes (c). Adapted with permission from Reference 6.
The heart has many other cell types, including inflammatory, smooth muscle, and connective tissue cells. The behavior of these cells can also be visualized using fluorescent labels, revealing complex interactions. In response to a laser-induced injury, resident macrophages, a type of immune cell, respond rapidly by extending processes toward the injury site (Figure 4a). The site is also marked by the leakage of blood plasma from capillaries. Lesions lead to altered contractility of adjacent cardiac muscle cells and changes in local tissue motion during the heartbeat (Figure 4b).
Multiphoton microscopy provides novel contrast mechanisms in addition to fluorescence. Three-photon microscopy with 1700-nm excitation enables deeper imaging of cardiac blood vessels compared to two-photon microscopy because of increased light penetration (Figure 4c). The longer wavelengths produce bright, second- and third-harmonic generation signals (Figure 4d)7,8. In harmonic generation, multiple photons interact with structures without absorption, producing radiation at exactly one-half or one-third of the exciting wavelength. These endogenous signals are visible without additional fluorescent markers. Collagen, such as in the external layer of the aorta, produces second-harmonic generation. Fatty deposits, including atherosclerotic plaques inside the aorta, produce third-harmonic generation. Future studies could use endogenous signals, in addition to fluorescent labels, to study disease mechanisms and search for therapeutic strategies.
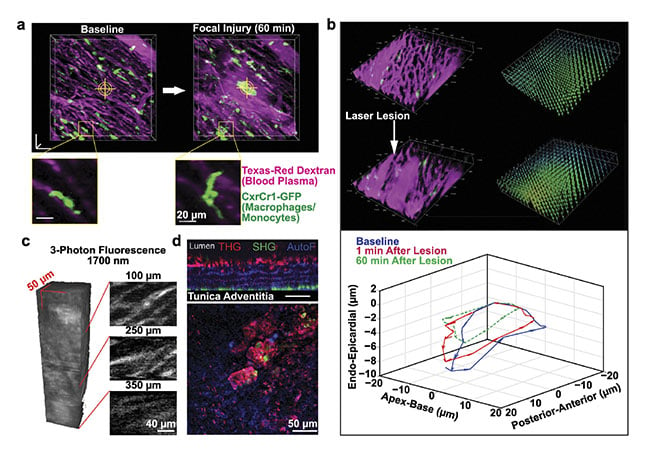
Figure 4. Markers of inflammation and cell pathology imaged by intravital microscopy. Resident macrophages respond to a localized laser ablation injury by extending their processes toward the injury site (a). Lesions can alter the contractility of the nearby cardiomyocytes (b). Three-photon microscopy enables deeper imaging of cardiac blood vessels compared to two-photon microscopy (c). Multiphoton microscopy generates endogenous signals such as third-harmonic generation from atherosclerosis (d). Image (a) adapted with permission from Reference 7; images (b) and (c) courtesy of Cornell University; image (d) adapted with permission from Reference 8.
More than in any other organ system, technical challenges remain for using multiphoton microscopy for cardiac studies. Two-photon microscopy allows visualization of the upper 200 µm of the myocardium, limiting imaging to the epicardial layer. The endocardial layer below, which is more sensitive to reductions in blood flow and oxygen, is often the first site of pathology and remains unexplored.
Future technological extensions include deeper imaging using three-photon microscopy, which is currently limited to slow frame rates by the lack of high pulse rates (10 MHz or more) in high-power lasers. Current studies use windows implanted at the time of imaging, and the mouse is imaged at only one time point. The ability to implant long-term windows would enable repeated imaging over time, which would be particularly helpful for studying chronic conditions such as heart failure. Such advancements would offer unprecedented visualization of the progression of pathology as well as response to treatment. The many hurdles in imaging the heart have made progress slower than with other biological systems, but this also means that more discoveries are waiting to be made.
Meet the authors
Anne Buglione, DVM, is pursuing a doctorate degree in the Schaffer-Nishimura Lab in the Meinig School of Biomedical Engineering at Cornell University. She received her bachelor’s and veterinary degrees at Cornell. Her research interests are in comparative physiology, translational medicine, and lab animal welfare. Her doctoral work focuses on the development and use of novel imaging methods — such as multiphoton microscopy, laser speckle contrast, and optical spectroscopy — to quantify blood flow and oxygenation in mouse models of cardiovascular disease; email: [email protected].
Nozomi Nishimura, Ph.D., is an associate professor in the Meinig School of Biomedical Engineering at Cornell University. She received her doctorate in physics from the University of California, San Diego; email: [email protected].
Acknowledgments
The authors are grateful to their colleagues David Small, Nathaniel Allan-Rahill, Jason Jones, and Daniel Rivera for their contributions to the data and figures used in this article, and to Richard Joseph and Bruce Kornreich for their encouragement and support. They acknowledge funding from National Institutes of Health grants R01HL161512 and T32ODO011000.
References
1. V.L. Roger et al. (2011). Heart disease and stroke statistics — 2011 update: a report from the American Heart Association. Circulation, Vol. 123, No. 4, pp. e18-e209, www.doi.org/10.1161/cir.0b013e3182009701.
2. W. Li et al. (2012). Intravital 2-photon imaging of leukocyte trafficking in beating heart. J Clin Invest, Vol. 122, No. 7,
pp. 2499-2508.
3. C. Vinegoni et al. (2015). Imaging the beating heart in the mouse using intravital microscopy techniques. Nat Protoc, Vol. 10, pp. 1802-1819.
4. J.S. Jones et al. (2018). In vivo calcium imaging of cardiomyocytes in the beating mouse heart with multiphoton microscopy. Front Physiol., Vol. 9.
5. D.P. Kavanagh et al. (2019). Imaging the injured beating heart intravitally and the vasculoprotection afforded by haematopoietic stem cells. Cardiovasc Res, Vol. 115,
pp. 1918-1932.
6. F.K. Lee et al. (2021). Genetically engineered mice for combinatorial cardiovascular optobiology. eLife, Vol. 10.
7. N.H. Allan-Rahill et al. (2020). Intravital microscopy of the beating murine heart to understand cardiac leukocyte dynamics. Front Immunol, Vol. 11, No. 92.
8. D.M. Small et al. (2018). Label-free imaging of atherosclerotic plaques using third-harmonic generation microscopy. Biomed Opt Express, Vol. 9, pp. 214-229.