Clever innovation and disciplined engineering are helping to create better, smaller and less expensive flow cytometers.
GIACOMO VACCA, KINETIC RIVER CORP.
Cells are the building blocks of organisms. From yeast and bacteria to elephants and whales, cells are the fundamental units of both biological structure and function. They have long been the subject of intense study: to better understand how they work, to help develop safer and more effective drug compounds, and to serve as diagnostic proxies of disease.
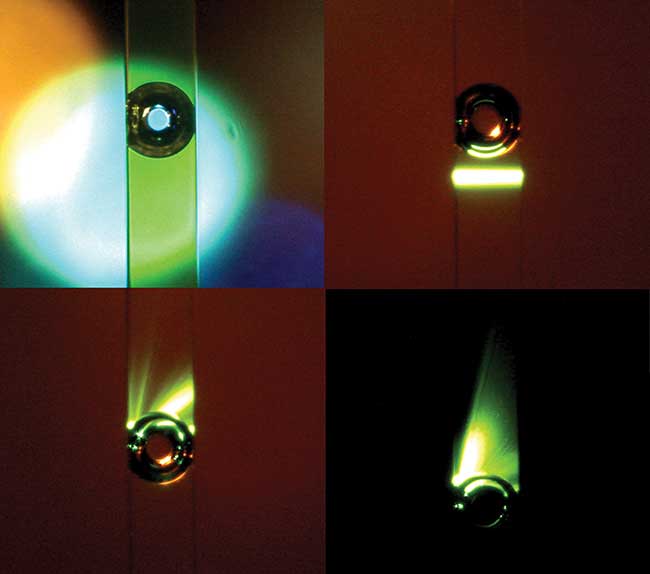
A large vapor bubble in a 250-µm flow cytometer microchannel caused by absorption of high-power green light by fluorescein dye. Bubbles large and small can create mesmerizing interactions with a laser beam, however they are very disruptive to precision cell measurements, and flow cytometer designers go to great lengths to prevent them. Courtesy of Giacomo Vacca.
Flow cytometry is often compared to microscopy, since these two techniques are largely complementary. Microscopy generates supremely fine-scaled images of a sample; but it’s slow, taking up to seconds to minutes to image in detail just a handful of cells. Flow cytometry, by contrast, provides only a handful of measured parameters on each cell, but at rates of tens of thousands of cells per second. The main trade-off is detail vs. throughput.
Most flow cytometers work by funneling cells from a liquid sample into a narrow stream so they’re in single file, and passing them one by one at high speed through one or more interrogating laser beams (see Figure 1). The resulting interactions generate scattered and fluorescent light, and an analysis of these allows counting, identification and characterization of the cells in the sample.
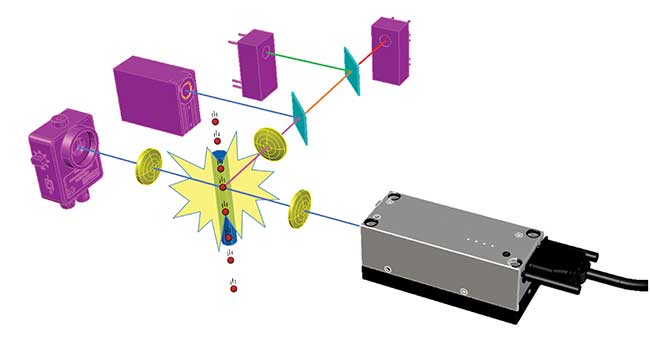
Figure 1. Principle of flow cytometry. Cells (red) flow one by one past the beam from a laser (gray), and the resulting light signals (various colors) are spectrally separated by filters (cyan) and detected by photodiodes and photomultipliers (pink). Image created using BeamWise modeling software. Courtesy of Giacomo Vacca.
AIDS diagnostics and hematology
Today, flow cytometers are ubiquitous in hospitals, clinical laboratories, cell biology departments, marine biology laboratories, biomedical research institutions and development labs of pharmaceutical companies.
One of the most remarkable success stories of flow cytometry is connected with the global fight against HIV/AIDS. As the epidemic grew in the 1980s, the need for a reliable diagnostic tool to help assess whether someone was getting sick from AIDS became critically important. HIV specifically targets CD4-positive T-lymphocytes (a kind of white blood cell), depleting them and making the patient vulnerable to infection. Flow cytometry can distinguish those cells from all other blood cells — quickly and reliably. The flow cytometry-based test thus became the worldwide gold standard for AIDS diagnosis, and one of the key tools widely adopted in the global efforts to treat sufferers of the disease1.
An even more widespread application of flow cytometry is routine blood counting. Every time someone gets his or her blood drawn, whether for an annual physical, or to follow up on a treatment, that tube of blood gets sent to a lab, just downstairs or maybe a few hundred miles away, where flow-based hematology analyzers generate a complete blood count (CBC), which includes red blood cells, white blood cells and platelets, in less than a minute per sample. This most common of diagnostic tests is ordered hundreds of millions of times per year, worldwide.
Main applications and unmet needs
But for all the advances since its inception a half century ago, flow cytometry is constantly being pushed further.
The relatively elevated cost and complexity of traditional flow cytometers means that, generally, laboratories share them: Instruments are acquired by a central facility, and then allocated to users across a department, an entire hospital, or even from other institutions. This limits access, and requires expert operators for their operation and maintenance. Simpler, cheaper machines would obviate these problems and foster broader adoption.
Some applications, like infectious-disease testing in remote areas, require instruments to be even smaller, ideally portable and robust. Traditional flow cytometers (such as BD Biosciences’ 525-lb LSR II) have long been just the opposite — bulky, lab-bound and complicated to operate. A push has been underway to package flow cytometry in more compact and intrinsically more reliable units. For example, there is a great need for a field-deployable malaria diagnostics solution.
In some fields of research, requirements are bumping up against rather fundamental physical limitations. For example, in recent years it has been increasingly recognized that tiny particles (microvesicles and exosomes) shed from cells can tell us a lot about physiology, disease and recovery2. These particles (see Figure 2) can be even smaller than bacteria, however, and they are very challenging to detect on current flow cytometers. Instruments capable of reliably detecting and characterizing these extracellular vesicles would help translate this new area of research into clinical practice.
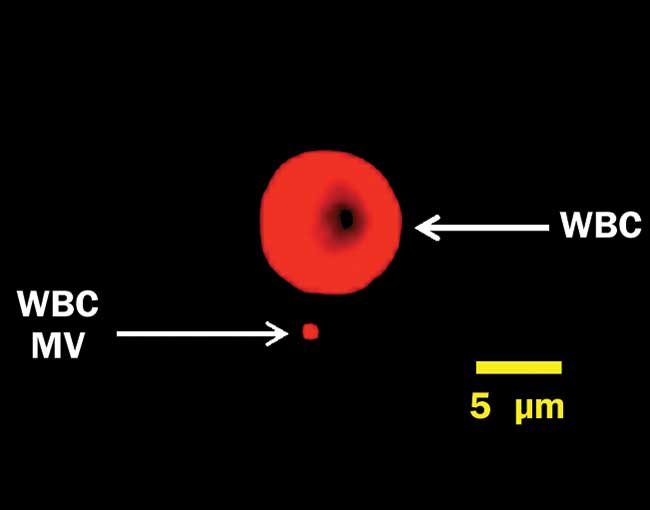
Figure 2. A white blood cell (WBC) and a microvesicle (MV), labeled for surface protein CD45 and captured on a Millipore ImageStreamX imaging flow cytometer under 640-nm excitation. This MV is near the resolution limit at 60× magnification. Courtesy of Joanne Lannigan, Flow Cytometry Core/Uta Erdbrügger, University of Virginia School of Medicine.
Another field of biomedicine constantly in need of greater capabilities is immunology. The study of the immune system and its myriad super-specialized “foot soldiers” (mainly white blood cells) requires an ability to distinguish cells based on their surface proteins3. Multiplexing (probing as many cell parameters as possible) is key: the more parameters probed simultaneously, the more detailed the picture of the cell population in a sample. But the complexity of running an instrument goes up exponentially with current multiplexing approaches, and traditional flow cytometers max out at around 20 parameters.
Robustness, ease of use, reliability
The calls for better, smaller, (and/or) cheaper flow cytometers are being answered by both clever innovation and disciplined engineering — and much of it is photonics-related. Early machines were little more than prototypes with a skin, and their legacy is easily seen in the design of instruments still in common use. More recently, design and engineering best practices from other industries have crept their way into flow cytometry, resulting in systems intrinsically less prone to environmental variations or operator error.
One of the first volleys in this ongoing competitive battle was the introduction of the C6 by Accuri, a Michigan startup later bought by BD Biosciences. The C6, launched in 2008, requires no laser alignment or maintenance by the user. More compact, robust and significantly cheaper than the then-available alternatives, it quickly gained traction in a market with significant pent-up demand for “simpler.” Research groups (including the author’s, then at Abbott Laboratories) started buying them for their own use, instead of having to rely on time-shared machines in the core labs.
A more recent step in this same direction was the introduction of the CytoFLEX, developed by Chinese startup Xitogen (bought in 2014 by Beckman Coulter). While the Accuri C6 is limited to two lasers and six parameters, the CytoFLEX (Figure 3) can support up to three lasers and 15 parameters, in a small footprint, and with performance that rivals machines many times its size. The CytoFLEX is regarded as being not just “smaller” (which often forces performance trade-offs), but “smaller and as good or better,” particularly in terms of fluorescence sensitivity4.

Figure 3. The three detection modules of a 3-laser, 13-color Beckman Coulter CytoFLEX flow cytometer. Each fiber-coupled module contains several compact dichroic filters on removable holders (like the one held up in the image) for spectral separation of signals. Courtesy of Beckman Coulter.
One of the photonics advances that is making possible this kind of improvement is the continued miniaturization of laser sources. For a long time, flow cytometers had to rely on massive, electricity-guzzling, water- or forced-air-cooled gas lasers, such as argon-ion and helium-cadmium. With the arrival, starting in the early 2000s, of solid-state alternatives, the footprint — both physical and carbon — of flow instruments could be significantly reduced. The latest laser products to hit the market (Figure 4) are as small as a pack of mints — enabling transformative instrument designs in terms of alignment stability and overall size.

Figure 4. Compact latest-generation solid-state lasers (see penny for scale), from Pavilion Integration Corp.’s Mini-Whisper laser product line. Courtesy of Pavilion Integration Corp.
Portability for field diagnostics
The Accuri and CytoFLEX are good fits for labs short on bench space and in need of full-featured solutions. But they’re far from portable. There are many applications where only a few parameters are needed, but at low cost and a “back-of-a-Jeep” sturdiness, such as bringing AIDS and malaria diagnostics out of urban centers and into unelectrified villages in rural areas.
Partec GmbH, a pioneering German flow cytometry company acquired in 2013 by Japanese diagnostics powerhouse Sysmex, has long made it part of its mission to serve just that kind of a market and global-health need. Partec’s CyFlow line of compact, robust flow cytometers enabled deployment of AIDS diagnostics deep into the African countryside of areas hard hit by the epidemic.
Recently, several companies have developed innovative solutions that are even more compact and robust. For example, handyem Inc., a Quebec City-based spinoff of Canada’s National Institute of Optics (INO), introduced the HPC-100, a waveguide-based flow cytometer based on a prototype that actually flew on the International Space Station (See Figure 5). The handyem box is one of the most portable flow cytometers yet, and its interrogation design makes it intrinsically alignment-free5.

Figure 5. Canadian Space Agency astronaut Chris Hadfield, floating in the International Space Station, together with INO’s Microflow cytometer. The instrument was tested on the space station to pave the way for health monitoring of astronauts during space missions. Courtesy of Chris Hadfield/NASA.
Multiplexing for immunology
The immunologist’s constant quest for greater multiplexing drove the development of machines with more and more excitation sources and fluorescence detection channels, such as the BD LSRFortessa X-20, which supports up to five lasers and 20 channels. Adding lasers and fluorescence detection bands, however, reaches an effective plateau with instruments like the LSRFortessa. The diminishing returns come in the form of spectral crosstalk: neighboring channels receive unwanted fluorescence spillover, distorting results and adding noise. This is the effect of the relatively broad emission spectra of most organic fluorophores. Unmanageable crosstalk, cumbersome workflows and poorly reproducible results pose a limit to the number of dyes that can be used concurrently.
The introduction of mass cytometry by Toronto-based DVS Sciences, acquired by Fluidigm, was supposed to change all that. Based on mass spectrometry of rare-earth isotopes instead of fluorescence, the company’s CyTOF is capable of 35+ simultaneous parameters — ostensibly without any of the compensation headaches of fluorescence-based flow cytometers. The CyTOF has found a receptive audience in immunology6. However, mass cytometry is not without challenges — such as limited sensitivity, unanticipated channel-to-channel crosstalk, expensive reagents and equally cumbersome workflows. Not least, the cells in a sample are completely obliterated during analysis, precluding any sorting application.
At Kinetic River Corp., the research team is actively working on new approaches to multiplexing that are compatible with sorting. Preliminary results, using different excitation sources, including high-performance lasers from Toptica Photonics, are encouraging; the goal is to double or more the number of simultaneous parameters currently available for use.
Sensitivity for nanoparticles
The recognition that smaller-than-cells particles can be important harbingers of certain disease states, and diagnostic entities in their own right, has spurred the field to push the envelope in terms of sensitivity of detection. One approach is to label the microvesicles and exosomes (broadly, extracellular vesicles — EVs) with fluorophores and detect them using standard instrumentation (Figure 2). The challenge there is that EVs, due to their size, do not support the levels of fluorescence staining normally seen in whole cells. Therefore higher-sensitivity instruments are needed.
Another challenge is that, for many applications, fluorescent labeling is not a desirable option — for example, when wishing to sort a fraction of the EV population and conduct further tests on them. In that case, labeling can alter the state and function of the particles, compromising the results. Label-free techniques are then required.
The simplest and most widely used label-free technique is the oldest one in the book: elastic light scattering. In the size range of cells this is known as Mie scattering, but when looking at EVs, you get into the Rayleigh scattering regime. Scattering efficiencies plummet as size decreases, making it inordinately difficult to detect at high throughput particles smaller than 100 nm in size.
Various fixes are under development. Improvements in the optical design (reduction of stray light, increased collection of scattered light, reduction in electronic and system noise sources), increase in the available optical power from solid-state lasers, and signal-processing techniques are all being deployed in the push for detecting the smallest possible particles quickly and reliably. Some instruments addressing this problem include the A50-Micro from Apogee, the Beckman Coulter Gallios and the Small Particle Option on the BD Influx. This may well be the case where all-around solid engineering design and execution, rather than a magic bullet, wins the day.
The future of cell analysis
There is much to look forward to in biophotonics, and flow cytometry is no exception. The relentless evolution of optical components is enabling amazing feats in realms of light excitation, collection and detection, and their application to cell analysis. Photonic developments, pioneered in other areas of science and technology, but which are now being adopted in flow cytometry, include: supercontinuum and pulsed light sources; photonic integrated circuits; spectrally resolved detection using prisms and detector arrays; inexpensive, compact solid-state detectors such as silicon photomultipliers; and more. Biophotonics innovators are hard at work on analytical and diagnostics bottlenecks — there’s no shortage of important problems to solve. The prize is better health and saved lives.
Meet the author
Giacomo Vacca is an entrepreneur, optical physicist and inventor (19 issued patents). He has two Harvard degrees and an applied physics Ph.D. from Stanford University. Vacca is president of Kinetic River Corp., a Silicon Valley firm offering custom flow cytometers. He is also co-founder of design automation software firm BeamWise Inc. Email: [email protected].
References
1. H.M. Shapiro (2003). Practical Flow Cytometry, 4th Edition. New York: Wiley-Liss.
2. U. Erbrügger and J. Lannigan (2016). Analytical challenges of extracellular vesicle detection: A comparison of different techniques. Cytometry A, Vol. 89, p. 123.
3. S.P. Perfetto and P.K. Chattopadhyay, et al. (2004). Seventeen-colour flow cytometry: Unravelling the immune system. Nat Rev Immunol, Vol. 4, pp. 648-655.
4. R. Duggan (2014). University of Chicago Flow Blog. A first look at the Beckman Coulter CytoFLEX — Strong performance in a small box, http://ucflow.blogspot.com/2014/09/a-first-look-at-beckman-coulter.html.
5. G. Dubeau-Laramée and C. Rivière, et al. (2014). Microflow, a sheathless fiber-optic flow cytometry biomedical platform: Demonstration onboard the International Space Station. Cytometry A, Vol. 85, p. 322.
6. E. Tricot and M. Meyrand, et al. (2015). Evaluating the efficiency of isotope transmission for improved panel design and a comparison of the detection sensitivities of mass cytometer instruments. Cytometry A, Vol. 87, p. 357.