Researchers produce new red and green fluorescent proteins.
Hank Hogan, Contributing Editor
Fluorescent proteins are essential tools for probing and understanding biochemical processes as they happen. And as any mechanic or handyman knows, you have to have the right tool for the job. That is why research into the development of new fluorescent proteins continues and is so important. A look at the efforts of three groups shows that some of the latest advances involve a new and brighter red fluorescent protein, a simpler but more versatile green fluorescent protein and the linking of optical output to structure. Such advances could have a significant impact on future biological research.
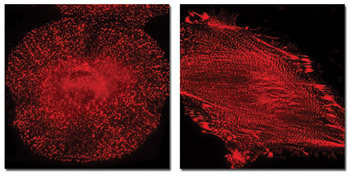
These images of human HeLa cells labeled with mKate, a new monomeric far-red fluorescent protein, are of clathrin (left) and actinin (right). Courtesy of Michael Davidson of Florida State University and Dmitriy Chudakov of Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry in Moscow.
Most people go to the pet store to pick up food for Fido or maybe to check out the fish. At Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry in Moscow, researchers do so to discover new fluorescent proteins. A few years ago they found an unusual red anemone in a pet store, recalled research scientist Dmitriy Chudakov. “This particular anemone, Entacmaea quadricolor, looked extremely bright.”
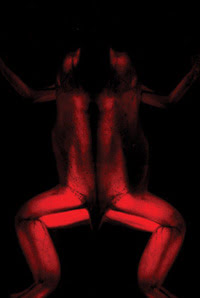
Expressing a new far-red fluorescent protein — Katushka — this frog glows in the far red when excited by light of the appropriate wavelength. Because of the wavelength of the light, it emerges from deeper within the tissue than shorter wavelengths. The researchers also developed a monomeric version of the protein named mKate. Courtesy of Dmitriy Chudakov, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Moscow.
Starting with the wild protein behind that brightness, Chudakov and his co-workers at the institute, along with researchers at Evrogen JSC of Moscow, used directed and random mutagenesis to create a new far-red fluorescent protein named Katushka. They also developed a monomeric version, mKate, with both high brightness and photostability.
In theory, fluorescent proteins enable the noninvasive labeling and tracking of specific cell types in living organisms in real time. Putting this into practice, especially for whole body imaging, is best done with fluorescent proteins with emission peaks in the 650- to 1100-nm range. Absorption by tissue and water is at a minimum in this window, and light scatters less at shorter wavelengths. For the same reasons, the excitation peak also should lie within this range or be as close to it as possible.
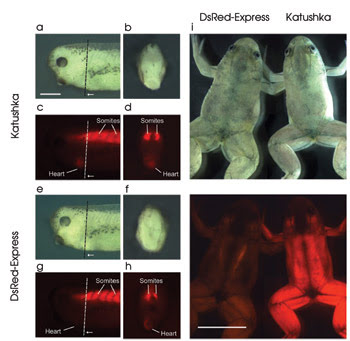
The researchers compared Katushka with DsRed-Express. On the left are fish embryos expressing Katushka (a–d) or DsRed-Express (e–h) under the control of a cardiac actin promoter. White light (a,b and e,f) and fluorescence (c,d and g,h) images are of the anterior (a,c,e,g) or head of the corresponding embryo (b,d,f,h). Dotted lines designate cut, with direction of view indicated by arrows. Scale bar is 0.5 mm. On the right (i) is a white-light (top) and fluorescence (bottom) image of 2.5-month-old frogs expressing DsRed-Express (left) or Katushka (right) from the dorsal side. As can be seen, Katushka is much brighter. Scale bar is 10 mm. Reprinted from Nature Methods with permission of Dmitriy Chudakov, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Moscow.
Researchers have been developing and investigating red and far-red fluorescent proteins, but those produced so far have been lacking. Some are dim, with a brightness only a tenth that of enhanced GFP. Others have an emission peak at too short a wavelength. Many have excitation peaks in the green, making effective excitation deep within tissue difficult.
In a quest to engineer a far-red fluorescent protein closer to the ideal, the Russian researchers began with some knowledge of the amino acids that surrounded the chromophore. They used this information to introduce promising changes, creating three libraries of proteins expressed in cells, then randomly mutated the hundreds of variants in each library.
“We consider this way the most efficient. It is a combination of rational selection of positions and random mutagenesis for the overall optimization, with a chance for luck,” explained Chudakov.
Indeed, he noted that the 203Arg mutation that ended up in the final protein was the result of just such luck. The scientists found the final protein by screening thousands of possibilities using an Olympus microscope with a built-in spectrophotometer. They used an excitation filter from 520 to 620 nm and a long-pass emission filter of 650 nm. After selecting the best variant, they again went through random mutagenesis optimization for four cycles, with Katushka coming about as a result.
Tests showed that the protein had a brightness equal to 67 percent that of enhanced GFP, an emission maximum of 635 nm and an excitation peak at 588 nm. At wavelengths greater than 650 nm, it was nearly twice as bright as any other far-red fluorescent protein.
The researchers looked at cell lines and developing frog embryos that had been transfected to express the new protein and found that the protein matured quickly and produced no abnormalities.
Conversion to monomer
As detailed in the September issue of Nature Methods, the wild-type protein turned out to be relatively easy to convert to a monomer. In this form, it could be widely applied to label cellular proteins. Through four direct mutations, the researchers transferred the spectral properties of Katushka to a monomer derived from the wild protein and produced mKate, which was 67 percent as bright as Katushka. Photobleaching studies done with a Leica microscope indicated that both the monomer and the dimer proteins were photostable.
Chudakov said that many groups are working with the new proteins. “We expect the first independent results to be reported in a year’s time.” He added that the rights for further distribution of the proteins belong to Evrogen.
When remodeling a home, you need to understand how things are put together and what role the different parts play. Otherwise, it is possible to remove a load-bearing beam and have the entire structure come down.
In their quest to engineer better fluorescent proteins, researchers also must know how things are put together and how various parts play different roles. Using x-ray and optical measurements, a group from Arizona State University in Tempe, from the University of Arizona in Tucson, and from Vanderbilt University in Nashville, Tenn., looked at the structure and properties of the Cerulean green fluorescent protein.
The researchers discovered something unexpected, said Rebekka M. Wachter, associate professor of chemistry and biochemistry at Arizona State University. The protein design strategy that generated the Cerulean variant was based on the idea that a carboxyl group in the protein would be exposed to a solvent. As revealed by x-rays of the protein’s structure, the carboxyl group instead is hidden and buried.
That finding, noted Wachter, can explain some of its observed optical properties, in particular a blueshift in absorbance of 10 to 15 nm at low pH. “We now think that the carboxyl group plays the role of a pH-dependent switch that controls the conformational state of the chromophore.”
In this model, the carboxyl-bearing side chain reorients itself when moving from an environment of high to low pH. This forces a rearrangement of the internal packing of the protein, favoring the cis conformation of the chromophore over the trans state. The result of this movement would be the blueshift in absorbance.
To arrive at this conclusion, the investigators first had to study carefully the structure of the Cerulean protein. The protein they investigated had been engineered specifically for fluorescence lifetime imaging microscopy, which requires that the donor in a donor-acceptor pair have a monoexponential excited-state decay. Unlike the cyan proteins to which it is related, Cerulean does have that profile and has been successfully used. They wanted to confirm the protein’s structure because that would add to the understanding of the protein’s behavior.
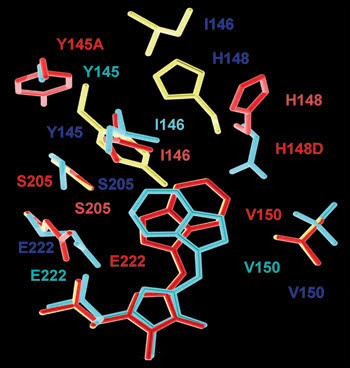
Researchers derived structural maps from x-ray measurements of crystallized fluorescent proteins. Here the differences in the chromophore environment are shown for Cerulean (red), cyan fluorescent protein minor (cyan) and enhanced cyan fluorescent protein major (dark blue). The structural overlay was produced by aligning the α-carbon positions of the proteins. These differences should explain and correlate to optical performance differences. Courtesy of Rebekka M. Wachter, Arizona State University.
To do this, they extracted, purified and crystallized the protein. They then used one of the brightest sources of soft x-rays in the world, the Advanced Light Source Beamline at Lawrence Berkeley National Laboratory in Berkeley, Calif., to take measurements that let them determine the structure of the protein.
To measure its optical properties, the scientists used a single-crystal spectrophotometer equipped with a CCD array detector from Spectral Instruments of Tucson, Ariz. They collected absorbance spectra of the protein crystals under cryogenic conditions for the wavelength range of 350 to 650 nm. This approach enabled them to make a direct comparison of the structure determined by x-ray with the optical properties of absorbance bands in the same set of crystals.
“Optical measurements on crystals are extremely important, since in solution, different conformations of the protein may co-exist in equilibrium with each other,” Wachter said.
These measurements showed that the carboxyl group was unexpectedly hidden and buried within the structure. The measurements also revealed other aspects that were not surprising. For example, the structure as determined by x-ray was consistent with a single conformation of the protein. That molecular arrangement explained the monoexponential fluorescence decay. The work is detailed in the Sept. 4 issue of Biochemistry.
As for the future, Wachter noted that researchers would like to understand better how fluorescent proteins that exhibit different optical properties have evolved. Natural selection has led to fluorescent proteins of many different colors, but the structural parameters that determine those colors are not entirely understood.
Uncovering and understanding such structural details and effects are of scientific interest. However, that knowledge also should enable researchers to better associate desired optical properties with particular structural parameters. “This correlation is extremely important in guiding our efforts to design better genetically encodable probes such as fluorescent proteins,” Wachter said.
The same results with less
What does it take to make Emerald green? If the question concerns the GFP that has been called the best of all versions, the answer is four amino acid substitutions — two less than the six substitutions actually present. Those are the findings of researchers from the Plant Biotechnology Institute and the University of Saskatchewan, both in Saskatoon, Canada.
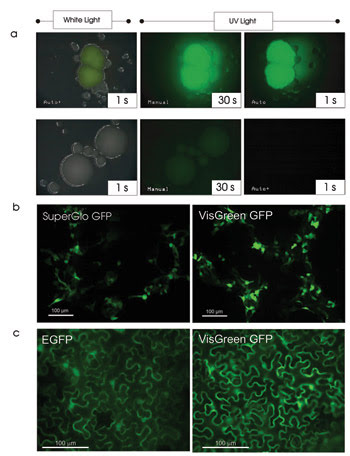
It is easier being green, thanks to a new fluorescent protein with fewer amino acid substitutions than others. VisGreen can be seen under white and UV light, expressed in bacteria at 1, 30 and 1 s, respectively, in the top images. Bacteria without any green fluorescent protein are shown for comparison (a). Human embryonic kidney (HEK293) cells express SuperGlo and VisGreen green fluorescent proteins with an exposure time of 1.745 s (b). Expression of enhanced GFP and VisGreen in plants with an exposure time of 1 s is shown (c). Courtesy of Gopalan Selvaraj, Plant Biotechnology Institute, Saskatoon, Saskatchewan, Canada.
The investigators used that information to construct their own GFP, which renders bacterial, plant and animal cells highly fluorescent, and which is virtually indistinguishable from Emerald.
The project that produced these results began years ago. The researchers were looking for the best GFP to use in plant cells, and they settled on Emerald. However, there was a problem in transfecting plants so that they would express the protein and fluoresce, recalled Gopalan Selvaraj, a research scientist at the Plant Biotechnology Institute. “There was no Emerald available with a codon bias for expression in plants.”
To correct this and to train students at the University of Saskatchewan in recombinant DNA manipulations, the researchers looked at various combinations of amino acid changes to the original GFP.
Investigators worldwide have tweaked the original protein to make many different variants, often doing so to correct a deficiency or to make a brighter version. For example, they have substituted amino acids to make versions that fold efficiently at higher temperatures because such folding is key to fluorescence intensity. Other changes have been made to improve the brightness when GFP is used with standard fluorescence microscopes, so that the excitation peak of the fluorescent protein is near 490 nm and the emission maximum is near 520 nm.
In the case of Emerald, there are six amino substitutions, although some reports put the number at five. In making a version of the protein that would be expressed in plants, the Canadian scientists found some intermediates that made recombinant Escherichia coli bacteria visibly green, even under dim room light. They took advantage of this result by examining the impact of amino acid substitutions found in Emerald on the brightness and performance of the GFP. They did so with the protein at body temperature, 37 °C.
Testing the candidate
In evaluating the results, they prepared a sample of a bacterial strain carrying a given GFP, with an equal density of cells in each sample. To measure the fluorescence, they used a PerkinElmer multiplate reader with continuous illumination at 485 nm and emission measurements from 530 to 540 nm. When imaging the cells, they used a Leica stereomicroscope with excitation and emission filters centered at 470 and 525 nm, respectively. From this data they calculated the intensity of the visible green color.
When making the amino acid substitutions, the team had a great deal of prior research available. “We knew what to expect up to a certain point,” Selvaraj noted.
In the end, they found that four amino acid substitutions, not six, produced a GFP with a quantum yield, molar extinction coefficient, folding efficiency, photosensitivity and relative fluorescence virtually the same as Emerald. In further tests, they showed that this new green fluorescent protein, dubbed VisGreen, worked well in bacteria as well as in mammalian and plant cells. Such studies have not been reported for either plants or animals in the case of Emerald. The work is detailed in the September issue of Biochimica et Biophysica Acta.
In summing up the project and future prospects, Selvaraj said, “For now, the marker is available to the research community for use. Making further improvements is a possibility.”