
KARL STORZ Launches Blue Light Bladder Cancer Treatment
Following the approval of a supplemental new drug application and a premarket approval supplement from the U.S. Food & Drug Administration, KARL STORZ Endoscopy-America Inc. has launched the Photodynamic Diagnosis (PDD) Blue Light Flexible Video Cystoscopy system for enhanced detection of nonmuscle invasive bladder cancer (NMIBC).
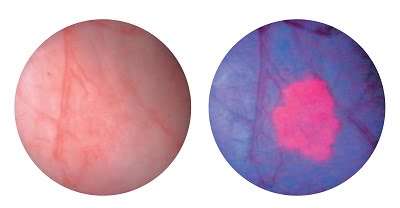
The Photodynamic Diagnosis (PDD) Blue Light Flexible Video Cystoscopy completes the two-part system, which also includes rigid blue light cystoscopy. Blue Light Cystoscopy with Cysview enables cancerous tumors to fluoresce in a bright pink color, improving tumor visibility and enhancing florescence-guided resection. Courtesy of KARL STORZ.
The FDA approval also extends the indication for Blue Light Cystoscopy with Cysview (BLCC) to include use of the new KARL STORZ PDD Blue Light Flexible Video Cystoscope. The approval includes an expanded indication for the repetitive use of Cysview within the same patient and for the identification of carcinoma in situ (CIS), one of the most challenging types of bladder cancers to detect. These new indications greatly increase the treatment possibilities of this therapy, which has rapidly become the standard of care for bladder cancer treatment at major medical institutions across the U.S.
Bladder cancer affects more than 708,444 patients in the U.S. Treatment is difficult because NMIBC tumors can look similar to normal healthy tissue and can be missed or incompletely removed. Unfortunately, patients have a high probability of cancer recurrence, and in 2018 it is estimated that there will be 17,240 patient deaths due to bladder cancer. The PDD Blue Light Flexible Video Cystoscopy completes the two-part system, which also includes rigid blue light cystoscopy. The flexible and rigid systems are designed to improve the visualization and resection of deadly NMIBC tumors. The new flexible system expands upon the rigid platform, enabling comfortable, anesthesia-free examinations to confirm suspected lesions from a previous cystoscopy and for ongoing monitoring of NMIBC.
The FDA approval is based on results from a large Phase III study using the KARL STORZ blue-light-enabled rigid and flexible cystoscopes and blue light video system.
"It is also the only FDA-approved technology shown to improve detection of NMIBC tumors, leading to improved and more comprehensive florescence-guided tumor resection,” said John Martineau, director of urology marketing for KARL STORZ. “In the 2016 guidelines for the management of NMIBC, the American Urological Association and Society for Urologic Oncology included blue light cystoscopy as recommended for increasing the detection and reducing recurrence of NMIBC. We are certain that use of blue light cystoscopy with Cysview will be positioned to provide enhanced diagnostic and treatment capabilities to physicians and support improved outcomes for patients.”
KARL STORZ Endoscopy-America is an affiliate of KARL STORZ SE & Co. KG, a developer of reusable endoscope technology.
/Buyers_Guide/Karl_Storz_Endoscopy-America_Inc/c24537