Benjamin Abrat, Mauna Kea Technologies Inc., and Andrew Masters, Coherent Inc.
Endoscopy is a minimally invasive procedure used to screen patients
and to investigate a wide variety of suspected health issues. It often involves
a biopsy for subsequent laboratory analysis with various methods that include visual
microscopy. However, the approach often fails to spot precancerous problems and
small cancerous growths.
Doctors would like to spot precancerous tissue
and small malignancies at their earliest, most treatable stage of development. A
new technology is helping to accomplish this. It allows an endoscope to perform
in situ, high-resolution fluorescence microscopy imaging in real time, at the cellular
and subcellular levels, via an instrument small and flexible enough to reach all
the way into the tiny alveoli in the lungs.
Cancers, such as tubular adenoma in
the colon, often are preceded by a curable, noninvasive stage that progresses asymptomatically
for several years before reaching an invasive stage. In many cases, the process
begins with the formation of anomalous cells in the deepest layer of the epithelium,
directly above the basal membrane that separates the epithelium from the deeper
layers of the tissue and that provides very strong and effective protection. Because
there are no blood vessels in the epithelium, epithelial cells cannot spread to
other parts of the body; hence, it is important to detect anomalies at a very early
stage, before the cancer breaks through the basal membrane to become invasive.
Researchers and clinicians have recognized
that in situ microscopy, in conjunction with endoscopic procedures, would help diagnose
disease earlier. For example, in suspected cases of Barrett’s esophagus, a
condition that can lead to cancer and that often is associated with long-term gastroesophageal
reflux disease, the goal is to look for evidence of dysplasia — a precancerous
change in tissue characteristics. This type of exam is typically an endoscopic procedure
that could involve excising 30 different samples. The ability to perform optical
biopsy could reduce this number, enable better targeting and, ideally, identify
suspected dysplastic tissue that otherwise would be missed.
Bronchoscopy, another endoscopic method,
is used to diagnose problems with the airway or to remove an object or growth from
the airway. For this procedure, biopsy forceps often are used to take samples in
the proximal lung. However, those samples frequently are not well targeted. In the
distal parts of the lung, smaller ducts and alveoli cannot be penetrated by traditional
endoscopes even if they have diameters of several millimeters. In these instances,
a flush-and-retrieve procedure called bronchoalveolar lavage is used to collect
loose cells for lab analysis with only limited location specificity.
Confocal microscopy
Laser scanning confocal microscopy is an attractive
endoscopy adjunct for several reasons. It yields a high signal-to-noise ratio, produces
images with high spatial resolution and can acquire surface or intracellular sectional
images. It also is compatible with a variety of specific or broad-use fluorophores
(introduced either endoscopically or intravenously) as well as with natural fluorescence.
From a practical standpoint, laser light can be easily delivered via optical fibers,
and suitably compact and rugged solid-state blue lasers are readily available for
integration into a turnkey system.
Although researchers have attempted
to miniaturize confocal microscope scanners and image-acquisition systems into the
distal tips of endoscopes, the diameter of these scopes has limited their use to
larger cavities, such as the lower gastrointestinal tract.
Mauna Kea Technologies Inc. of Cambridge,
Mass., has taken an alternative approach with its Cellvizio system. It uses fiber
optic bundles and image-processing software to place the confocal laser scanning
mechanism at the proximal end of the endoscope; i.e., outside the body. This has
enabled the development of Fluorescence Confocal Miniprobes with an overall diameter
of 1.4 mm (for lung endoscopy) or 2.5 mm (for gastrointestinal endoscopy). The probes
are pliable enough to be inserted through the existing working channel of any conventional
flexible endoscope. For research purposes on small animals, overall bundle diameters
as small as 300 μm are available.
The heart of the system is the laser
scanning unit (Figure 1). The majority of applications require blue excitation,
provided with a Sapphire laser from Coherent Inc. The reliable and low-noise laser
has a 22-mW, 488-nm output. It uses optically pumped semiconductor technology that
is fully power- and wavelength-scalable, simplifying future product development
and compatibility.
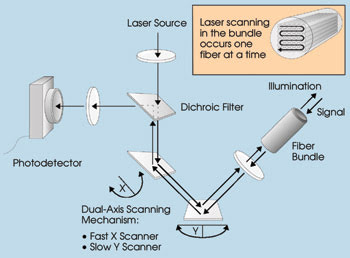
Figure 1. The scanning performed by
this fiber optic confocal endoscopy system is done outside the body.
In the system, the laser beam is deflected
with a pair of scanning mirrors before passing through a positive lens matched to
the numerical aperture of a fiber bundle.
The fused fiber bundle contains up
to 30,000 individual fibers with a core diameter of 1.9 μm and an average intercore
spacing of 3.3 μm. With this arrangement, the beam is rastered over the input
facet of the bundle at up to 12 scans per second. Because this is a coherent fiber
bundle, the spatial mapping of the laser across the thousands of individual fibers
is completely preserved at the distal end, even when the fiber bundle is several
feet in length.
Returned fluorescence follows the same
path via the scanning mirrors and is spectrally filtered to eliminate laser backscatter
before being focused onto an avalanche photodiode through a confocal aperture.
The fused bundle is clad in a protective
sleeve, allowing it to be fully decontaminated after each use in a chemical bath
of the type routinely used for flexible endoscopes. At the distal end of the bundle,
several termination options are possible (Figure 2).

Figure 2. The fiber bundle can terminate with no additional optics
or with a positive lens to enable confocal imaging at a finite depth.
In the simplest format, the bundle
terminates in a cleaved end that is surrounded by a thin metal ferrule. The ferrule
protects the fiber tip and enables it to perform soft-contact surface imaging of
tissue by eliminating the danger of scratching or piercing that could occur with
a bare glass bundle. This arrangement is used where surface images are required.
X-Y discrimination is provided by the fiber bundle; only fluorescence in front of
the excitation fiber(s) is returned through that fiber to the detector.
A weak level of Z-axis discrimination
is provided by natural attenuation; both the laser excitation and resulting fluorescence
are absorbed and scattered by cellular material, favoring fluorescence detection
at or near the cellular surfaces. The narrow dimensions and the numerical aperture
of the individual fibers also strongly favor detection of only near-field fluorescence.
With this configuration, the system delivers lateral or X-Y resolution of ~5
μm, with the Z-axis signal representing an integration of ~200 μm
of surface depth.
Reimages, demagnifies
Alternatively, the fiber bundle can be terminated
with a positive lens that provides higher X-Y resolution because it re-images and
demagnifies the fiber bundle at a finite depth with the tissue. Just as important,
this configuration also delivers confocal Z-axis (depth) discrimination. Here, the
aperture of each fiber acts as a confocal aperture so that out-of-focus fluorescence
is not efficiently coupled into that fiber. Two termination lenses are offered,
one to image a 30-μm layer near the surface and the other to deliver images
from 70 to 90 μm below the tissue surface. Maximum X-Y resolution in these
latter images is around 2.5 μm.
The unique optical configuration of
the system requires some specialized image-processing techniques. Scanning is performed
by fast horizontal sweeping (4 kHz), accompanied by slow vertical stepping, for
a total frame rate of 12 Hz. (Because the horizontal scanning has a sinusoidal velocity
profile, only the central portion of the trajectory is used, where the horizontal
velocity is nearly linear.)
The raw images essentially are confocal
images of the proximal end of the fiber bundle, and thus modulated by the fiber
pattern. To permit a smooth image reconstruction, the bundle is oversampled so that
the raw data shows the individual fibers in the bundle.
This first part of signal processing
also corrects for any fiber-to-fiber variations and eliminates all bundle artifacts.
The images are displayed on the system monitor with a resolution of 500 x 400 pixels
(Figure 3).
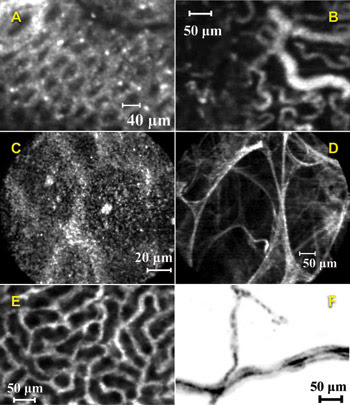
Figure 3. These single-frame images acquired with the Cellvizio system
show an in vivo mouse colon after instillation of acriflavine (A), in vivo tumoral
angiogenesis in a mouse labeled with FITC-dextran (B), in vivo reflectance imaging
of human mouth mucosa (C), ex vivo autofluorescence imaging in a human lung (D),
microcirculation of the peritubular capillaries of a live mouse kidney with FITC-dextran
(E), and dendritic receptors in a live Thy1-YFP mouse (F). (A) courtesy of Danijela
Vignjevic et al, Institut Curie; (B) courtesy of Anne-Carole Duconseille and Olivier
Clement, Université Paris V; (D) courtesy of Pierre Validire, Institut Mutualiste
Montsouris; (F) courtesy of Igor Charvet et al, BioCell Interface.
In many instances, however, it is useful
to view a wider field by combining a series of images obtained as the probe is moved
across the tissue surface. A unique set of mosaicking algorithms is used for this
purpose. These complex manipulations correct and combine the images using three
main steps:
First, the images are registered and
stitched together in a reference coordinate system. Then the software corrects each
frame for motion of the probe. Here, the line scanning is assumed to be instantaneous,
whereas the vertical scanning occurs within the timescale of the probe motion; the
actual observed area thus being more often a parallelogram than a rectangle. Finally,
frames are corrected for the slight tissue distortions produced by the pressure
of the moving probe.
Besides yielding an extended field
of view, this process delivers a final image resolution that is higher than that
of the original individual frames. Figure 4 shows how the image quality successively
is increased by these three steps for a mosaic of 50 in vivo images of murine colon.
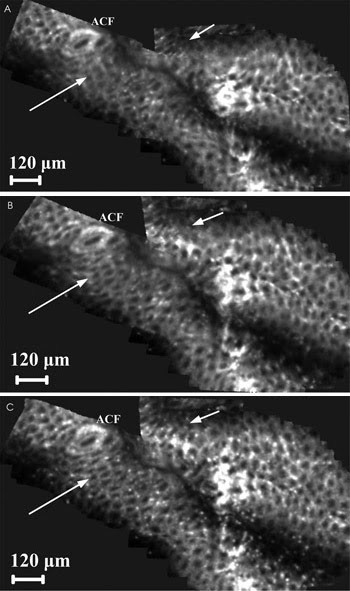
Figure 4. Mosaics of five in vivo mouse colon images after instillation
of acriflavine showing three levels of image mosaicking: After assembly and registration
of rigid, uncorrected frames (A), after correction of motion (B) and after correction
of nonrigid tissue distortion (C). ACF = aberrant crypt foci. Courtesy of Danijela
Vignjevic et al, Institut Curie.
Expanding applications
The endoscopic system is in clinical trials at
several facilities worldwide and already has been cleared by the US Food and Drug
Administration for bronchoscopy as well as for gastrointestinal endoscopy. As a
clinical diagnostic tool, this technology bridges the traditional gap between macroscopic
in vivo imaging tools such as MRI and PET, which have limited spatial resolution,
and high-resolution microscopy, formerly limited to ex vivo samples. We believe
that its main impact will be the enhancement of diagnostic capabilities for early-stage
cancer detection in the gastrointestinal and respiratory tracts as well as for interstitial
lung diseases in the distal airways (Figure 5).
In the area of laboratory-based animal
research, there already are many published studies using this technology. These
involve microendoscopic investigations such as colonoscopies in mice, in vivo imaging
of murine heart and lung tissue, and cell-to-wall interactions in murine arteries.
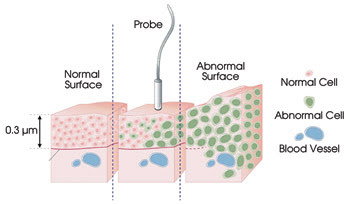
Figure 5. Many cancers begin as anomalous cells in the
epithelial
layer. Fiber optic confocal microscopy provides the ability to detect
these cells
before they penetrate the basal membrane and begin to migrate.
Another investigative area has been
tissue engineering. Here, the imaging technique has been used to check the quality
of labeled stem cells in vitro and to monitor the cells in vivo after implantation.
In other studies, the ability to provide
extended imaging in real time has been used to observe peripheral nerves and motor
end plates in rats over very long distances. And in deep-brain studies, the technique
has been used to monitor neuron precursor migration using time-lapse imaging over
periods as long as 50 minutes.
Meet the authors
Benjamin Abrat is vice president of business development
and strategic alliances at Mauna Kea Technologies Inc. in Cambridge, Mass.; e-mail:
[email protected].
Andrew Masters is director of marketing at Coherent Inc. in Santa Clara, Calif.; e-mail: [email protected].