The ability to watch biological processes unfold enables new discoveries in a range of fields from fertility to brain development.
Viewing biological processes as they happen provides a fascinating insight into cell behavior and can
unveil vital clues about developmental milestones that have been puzzling scientists
for some time.
Time-lapse microscopy (TLM) involves repeated capture of images
from a microscope at regular time intervals. The duration of the intervals determines
the temporal resolution, and the resulting video sequence shows cells or organisms
at work, giving scientists a first look at some important biological processes.
Demystifying embryo development
The earliest stages of human development remain largely a mystery,
despite the attention given to embryonic research. Determining the reasons why some
fertilized eggs go on to become healthy babies and why some stall and fail is still
one of the biggest questions facing fertility experts.
Now, a company founded by a team of scientists at Stanford University
School of Medicine in California is using TLM to predict with 93 percent certainty
which fertilized eggs will make it to a critical developmental milestone and which
will be unable to survive.
Auxogyn is a California-based company conceived by Renee Reijo
Pera, director of the Stanford University Center for Human Embryonic Stem Cell Research
and Education, to improve the effectiveness of in vitro fertilization (IVF).
One out of every six couples is affected by some degree of infertility,
according to the International Council on Infertility Information Dissemination.
With the rate increasing with maternal age and more women starting their families
later in life, the demand for IVF and other assisted reproduction diagnostic tools
is growing by around 10 percent each year.
The stark reality of assisted reproduction is that only one-third
of human embryos will develop successfully, according to the Centers for Disease
Control and Prevention in Atlanta. This often prompts the transfer of two or more
embryos to increase the odds of a successful pregnancy. However, if multiple embryos
implant and develop successfully, a woman and her physician may choose to selectively
abort one or more to increase the odds for the remaining embryos.
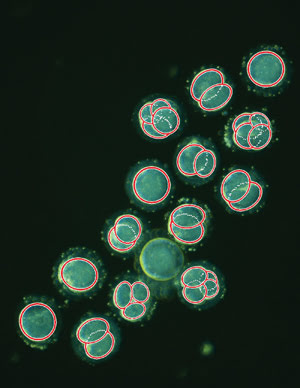
This is a single frame taken from a time-lapse sequence showing embryos
within the first two days after fertilization, with overlaid cell-tracking data
(red circles) produced using image analysis software. Courtesy of Kevin Loewke at
Auxogyn.
Identifying the embryo with the greatest potential would reduce
the cost associated with multiple IVF cycles; in addition, it would substantially
decrease the number of multiple births and significantly increase the success rates
of IVF.
Pera and colleagues at Stanford published their findings October
2010 in Nature Biotechnology. The researchers used TLM to take a closer look at
what happens during the first few days after an egg is fertilized. They followed
the cells through to the development of a hollow ball called a blastocyst, which
typically occurs within five to six days after fertilization and is usually an indication
of a healthy embryo.
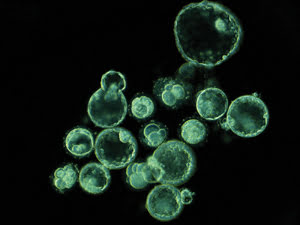
This single frame, taken from the time-lapse sequence on day five, shows embryos that
have arrested and embryos that have reached the blastocyst stage. Courtesy of Kevin
Loewke at Auxogyn.
“In our recent paper, we observed the developmental process
of early-preimplantation human embryos during the first six days of life using time-lapse
microscopy,” said Connie Wong, a co-author of the paper. “After performing
a thorough analysis of the imaging data, we successfully extracted three quantitative
parameters that can predict the developmental potential of an embryo before day
three of life.”
The team believes that these parameters can be used as diagnostic
markers in IVF clinics to aid in the selection of healthy embryos for transplantation.
Currently, most IVF clinics use only time-point analysis to determine embryo health
prior to transfer into patients. However, the Stanford study illustrates the importance
of measuring time-resolved events.
“When compared to a traditional time-point microscopy study,
in which researchers would only collect imaging data of their specimens at specific
selected time points, time-lapse microscopy offers the advantage of being able to
acquire data continuously during the time of the study,” Wong said. “This
is especially important when time-resolved data is needed, or when the specimens
tend to change frequently and unpredictably.”
Given the delicate environment required for human embryos to flourish,
the team had to develop a miniature time-lapse microscope so that the embryos could
be imaged inside a tissue culture incubator used to provide optimal culturing conditions.
Another crucial change in the typical TLM setup was the lighting.
Most of today’s TLM studies are performed using stand-alone microscopes illuminated
by standard bright-field or fluorescence illumination. Wong and colleagues, however,
switched to dark-field illumination to minimize the amount of phototoxicity experienced
by the embryos.
The research team at Auxogyn is now working toward commercializing
the miniature time-lapse microscopes for IVF clinical use.
“We are pleased to be starting a multisite clinical trial
soon that will further validate the effectiveness of the three time-resolved parameters
discovered in Stanford’s recent publication,” said Lissa Goldenstein,
CEO of Auxogyn. “We hope to introduce time-lapse microscopy with clinically
validated parameters as a routine tool used in assisted reproduction clinics to
better predict the developmental potential of embryos.”
TLM unlocks secrets of the brain
TLM is also breaking new ground in another area of human development:
neuronal circuits in the brain. Dr. David Solecki at St. Jude Children’s Research
Hospital in Memphis, Tenn., is using TLM to discover new details about mechanisms
regulating a crucial step in brain development. The study offers insight into the
origins of epilepsy, mental retardation and possibly brain tumor metastasis.
Dr. David Solecki at St. Jude Children’s Research Hospital is using TLM to reveal some of the secrets of brain development.
Courtesy of St. Jude Children’s Research Hospital.
For Solecki, time-lapse imaging offers an unparalleled glimpse
into those molecular and cellular mechanisms that are at the center of most biological
problems.
“Many investigators feel that time-lapse imaging does not
amount to much more than a set of pretty pictures that may not necessarily provide
any new insights into the biology of a particular process,” he said. “Contrary
to this sentiment, I’m convinced that time-lapse imaging provides an exciting
opportunity to directly observe a process to reveal how it mechanistically unfolds,
while at the same time directly quantitating the kinetics of that process as it
normally occurs, or when we manipulate it in a rational manner.”
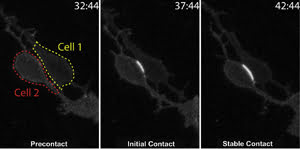
Time-lapse imaging tracks cell-to-cell binding for the first time. The cell borders are indicated
by red and yellow lines in the first frame. Subsequent frames show cell adhesion
indicated by a fluorescence signal, which intensifies upon establishment of stable
contacts. Time stamp = minutes:seconds. Image has been adapted from a paper in Science,
courtesy of Dr. David Solecki, St. Jude Children’s Research Hospital, Memphis,
Tenn.
The process in question for Solecki and his team is neuronal migration.
It’s been known for the past 15 years or so that neuronal migrations are essential
for proper brain formation; in particular, neuron cells must travel to specific
areas in the brain to complete necessary brain circuitry.
However, the major challenge has been to dissect the molecular
and cellular mechanisms controlling when and where neurons choose to migrate during
the assembly of brain circuitry. Solecki’s team suspected that how neuronal
cells adhere to neighboring cells plays a part in making those migration decisions.
In a paper published in Science on Dec. 24, 2010, the team used
TLM to dissect a signaling pathway that was believed to control the migration choices
of maturing neurons in the developing brain. The researchers developed a fluorescent
probe that, when combined with TLM, made real-time viewing of cell-to-cell binding
possible for the first time. They used a spinning disk confocal, which is ideal
for capturing dynamic events without overexposing the cells or brain slice.
“My laboratory has developed a variety of techniques to
genetically manipulate large numbers of neurons in brain slices or primary cultures
and image the cells in these preparations using a spinning disk confocal microscope,”
Solecki said. “Time-lapse microscopy was integral for us to determine that
the transition of one form of migration to another was related to the activation
of cell adhesion in mature neurons so they were able to adhere to a new migration
substrate as they move to their appropriate location in the brain.”
While he admits that TLM cannot replace the biochemical, anatomical
or molecular assays that are the mainstays of modern biology, he believes that the
technique can help shed light on the underlying mechanisms of particular biological
processes. All you need is the imagination to make these processes amenable to imaging
techniques.
“In the future, I hope to see time-lapse imaging as an alternative
to the static anatomical studies that are most frequently used today in the developmental
biology or neural development fields,” he said. “Given the ever-increasing
number of mouse models for human developmental disease (like those for neurodevelopmental
disorders or pediatric cancer), quantitative time-lapse imaging will be one tool
that will be very useful to unlock the cell biology and pathology of these diseases.”