Improvements in medical imaging promise leaps forward in diagnosing and treating diseases.
If the old saying is right and an ounce of prevention really is worth a pound of cure, then some of the latest medical imaging breakthroughs could represent tons of prevention and even more of cure. There are techniques with the potential to catch breast cancer while tumors are still small. Other methods allow doctors to touch the liver and other internal organs for a remote diagnosis. Still other medical imaging tools can improve biopsy results or track diseases of the eye with more precision. These methods show that, in the future, physicians will be seeing more of their patients than ever – to the benefit of both.
From both sides now
Although widely used and effective, mammography isn’t perfect. Its sensitivity is not sufficient for radiographically dense breasts – precisely the type of tissue that carries a significant risk for cancer.
An alternative is magnetic resonance imaging (MRI), which offers good sensitivity. However, the price of a bilateral breast MRI runs as much as 15 times that of a mammogram.
Now researchers at the Mayo Clinic in Rochester, Minn., have shown that an imaging technique based on relatively new cadmium zinc telluride (CdZnTe) single-photon detectors may provide similar sensitivity at considerably less expense. Lead investigator Carrie B. Hruska estimated that the cost for this type of imaging could be about one-fifth that of an MRI.
She explained that the sensitivity arises from the use of two detectors, each of which picks up the signal from an injected radioisotope that collects in tumor tissue. These detectors are positioned on opposite sides of a patient’s breast. The use of two detectors cuts in half the distance to the accumulated radioisotope, upping the signal and sensitivity. What’s more, the detectors allow clinicians to position the breast directly on the camera, much as is done with mammography.
“This optimal positioning, combined with the excellent spatial and energy resolution of the CdZnTe technology, permits the detection of very small cancers,” Hruska said.
An evaluation of 150 patients scheduled for biopsy showed that the dual-head gamma camera technique identified lesions less than 10 mm in size at an 82 percent rate. In this study, published in the December 2008 American Journal of Roentgenology, the researchers used prototype CdZnTe detectors from GE Healthcare of Chalfont St. Giles, UK, or from Gamma Medica-Ideas of Northridge, Calif.
Hruska led a subsequent study of 940 women with mammographically dense breasts and an increased risk of developing breast cancer, the interim results of which were presented at the 2008 American Society of Clinical Oncology Breast Cancer Symposium; the study showed that the dual-head technique has three times the cancer detection rate of mammography alone.
In addition to figuring out what role the technique can play in the breast cancer detection toolbox, the researchers have been working on improvements involving the camera’s count sensitivity, which could lead to several advantages. “This will allow us to reduce the necessary dose administered to the patient and the time it will take to acquire images,” Hruska said.
The latter could be an important benefit. Currently the technique takes 40 minutes to acquire images, which the researchers noted is probably near the limit of patient tolerance for remaining upright and still.
Getting to the right spot
While noninvasive procedures like MRI or mammography are preferred, sometimes only a biopsy will do. For that, physicians must guide a needle from outside the body to a precise point inside it. If they’re off, the biopsy doesn’t convey a true picture. Likewise, if they need to inject a drug or perform some other treatment at a particular location, the positioning of the needle must be precise.
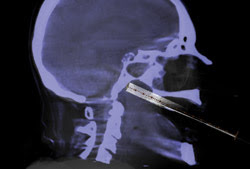
Shown is a needle biopsy through the mouth, with fluoroscopy registered (tube) to background angiography CT. Image courtesy of Philips Healthcare.
Philips Healthcare, which has its US office in Andover, Mass., offers a system for this precise positioning, a result of a technology that appeared a few years ago. Andrew T. Dunn, the company’s North American marketing director for cardiovascular x-ray, said that’s when angiography systems, which are used to image blood vessels, changed from image intensifiers to digital flat detectors. That enabled a much higher contrast ratio, with the 14-bit contrast depth allowing more than 16,000 levels of gray.
That greater contrast allowed the angiography systems to do more, a capability that Philips used by extending the x-ray-based imaging from fluoroscopy into computed tomography (CT). “We spin the radial gantry arm around the patient and do a cone-beam reconstruction to create a CT right in the angiography suite,” Dunn said.
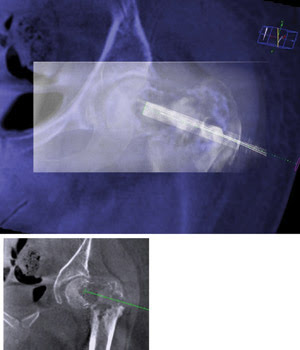
Fluoroscopy (rectangular box, top) registered to background angiography CT guides a bone graft target. The bottom box shows the needle planning for the procedure. Image courtesy of Philips Healthcare.
Sometimes called a “poor man’s CT,” this method doesn’t produce data with the same contrast resolution as seen in images from a dedicated CT machine, but it does have its advantages. For one, patients don’t leave the angiography suite, and their positioning doesn’t change. This stability makes it possible for software within the system to co-register and thereby overlay the data provided by the CT scan with information about where the needle is, which comes from fluoroscopy. In this way, physicians get live 3-D needle guidance. The approach, according to Dunn, shortens procedure times significantly, reduces risk and improves lab work flow.
At the Radiological Society of North America meeting in December 2008, Philips previewed a future enhancement that will allow the importing of previously acquired CT and MRI data into the system. That will require low-dose x-rays to pinpoint the location of natural anatomic landmarks, but the imported data can show structures not visible on the angiography CT.
Remote touch
Of course, sight isn’t the only sense; take touch, for example. A healthy liver feels soft. One developing fibrosis is firmer, and one ravaged by cirrhosis, or advanced liver disease, can be rock-hard. Doctors, unfortunately, can’t readily touch the liver or a great many other organs.
That inaccessibility to touch, however, may be a thing of the past. Researchers at the Mayo Clinic have been investigating magnetic resonance elastography, or MRE. The technique gauges the stiffness of tissue remotely, takes only seconds and is noninvasive.
In MRE, clinicians strap a small device around a patient’s abdomen before he or she enters an MRI machine. Once inside, the device gently vibrates the abdomen, sending low-frequency mechanical waves through the patient. Internal organs, including the liver, alternately expand and contract. These small movements are tracked with the MRI, yielding valuable information about the elasticity of the tissue.
In November 2008, at a meeting in San Francisco of the American Association for the Study of Liver Diseases, Jayant Talwalkar, a Mayo Clinic hepatologist, presented an investigation of 113 patients whose livers were diagnosed via MRE. The study revealed that the technique detected cirrhosis 88 percent of the time that a biopsy did. With MRE, doctors correctly identified patients with nonalcoholic fatty liver disease and no significant inflammation or fibrosis with 97 percent accuracy.
If successful, MRE will allow some patients to avoid biopsies, which involve the insertion of a needle and extraction of tissue. Besides their invasiveness, another problem with biopsies is that they miss or underestimate liver fibrosis, which frequently – in 20 to 30 percent of cases – has a patchy distribution.
MRE is currently used at the Mayo Clinic to detect hepatic fibrosis in patients with known or suspected chronic liver disease. Elastography also could be useful in imaging and diagnosing other ailments, with the applicability of MRE to Alzheimer’s disease and cancer currently under investigation.
Capturing the movement of tissue in response to sound waves also doesn’t have to be done using an MRI. For example, the endoscopic version of the technique uses ultrasound imaging. Michael B. Wallace, a gastroenterologist at the Mayo Clinic, noted that endoscopic ultrasound elastography could have an important role, if and when it’s ready to be deployed. “I see it evaluating lymph nodes and directing fine-needle aspiration to those lymph nodes likely to contain cancer.”
Getting more than a pretty picture
Finally, many medical imaging technologies present a common challenge. They can generate an overwhelming amount of data, making data manipulation tools vital if anything more useful than a stunning image is to be produced.
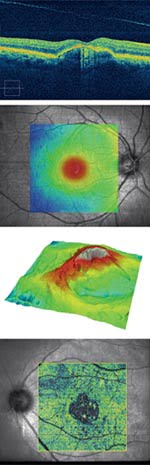
Retinal optical coherence tomography helps image eye conditions like age-related macular degeneration (a), central serous chorioretinopathy (b), epiretinal membrane (c) and vitreomacular traction with geographic atrophy (d). Images courtesy of Carl Zeiss Meditec.
An example of this can be seen in the fourth-generation retinal optical coherence tomography, or OCT, product from Carl Zeiss Meditec of Dublin, Calif. The system features spectral domain OCT, which acquires data 65 times faster than the technology used in previous products.
The company had to decide what to do with that increased data rate, said Scott Meyer, Zeiss Meditec’s director of strategic business development. “What we have chosen to do is to take full advantage of the speed to generate higher-definition maps by collecting volumetric data sets.”
The Zeiss products are aimed at ophthalmic applications. They allow physicians to measure the retinal nerve fiber layer around the optical nerve head, thereby gauging the progress of glaucoma and a patient’s response to treatment. The devices also are being applied to age-related macular degeneration, a leading cause of blindness.
Meyer noted that the mountains of data generated must be processed and presented in such a way as to be clinically useful. Thus, the system’s software has segmentation algorithms that allow doctors to sift rapidly through the image data and find what’s important.
In speaking of the future of OCT and its application, he predicted better hardware and systems. But that’s only part of the story, Meyer said. “What is just revolutionizing this space is how you help doctors to manage the data.”
Snapshots of cells under attack
Although they look the same, white blood cells, or primary B cells, respond to invaders differently. That’s no surprise, given that there are billions of combinations of antibodies on such cells that bind to bacteria, viruses or toxins. Because of this diversity and the small sample sizes of the cells, researchers have found it challenging to map specific antibodies to the genes that encode them. That’s made the evaluation of responses to vaccines or infectious disease difficult at the cellular level.
Now a new imaging technique could change that, said J. Christopher Love, an assistant professor of chemical engineering at Cambridge-based MIT. “This approach provides the physical linkage between the antibodies produced by certain cells and the cells themselves – the carriers of the genes.”
Love helped develop the method while at the lab of biology professor Hidde L. Ploegh. The technique uses an array of microscopic wells to segregate individual cells into 0.1-nl volumes. The cells secrete antibodies that are captured on the surface of a glass slide that is pressed up against the microwell array. The captured antibodies can be used to print out the response pattern, with fluorescent reagents making the pattern visible to microarray scanners.
Knowing which cell produced what antibody allows mapping of the response of the genes. In a study that appeared in the Nov. 18, 2008, Proceedings of the National Academy of Sciences, Love, Ploegh and their co-workers subjected mice to a series of immunizations designed to mimic a multipart vaccination, then characterized the response of their cells. The technique allowed the researchers to take a snapshot of the cells’ diverse reactions to the process.
That data could someday provide significant information. “By mapping the human immune response, the efficacy of new vaccines may be gauged accurately,” Ploegh said.
Today, vaccines that are given in multiple booster injections follow a carefully choreographed sequence, even though the doses administered later may be needed sooner, later or not at all. The cell-printing technique could be used to improve this process. Ploegh said that the group has had inquiries about commercializing its technique. He predicted that development of a device based on the method would take several years.