University of Rochester researchers have found that the use of a metal substrate can boost the photoelectric conversion efficiency of perovskites. Perovskites, a family of crystalline materials, have shown promise as a far less expensive and equally efficient replacement for silicon in solar cells and detectors. Typically, the material is synthesized in a wet lab and then applied as a film onto a glass substrate.
The Rochester researchers, led by professor Chunlei Guo, devised a method that, rather than glass, uses a substrate of either a layer of metal or alternating layers of metal and dielectric materials.
Guo and his team found that the architecture boosted the perovskite’s light conversion efficiency by 250%.

Researchers at the University of Rochester used a metal substrate to boost the photoelectric conversion efficiency of perovskites by 250%. The novel perovskite scheme was tested underneath simulated sunlight to determine its efficiency. Courtesy of J. Adam Fenster/University of Rochester.
“All of a sudden, we can put a metal platform under a perovskite, utterly changing the interaction of the electrons within the perovskite. Thus, we use a physical method to engineer that interaction,” Guo said.
In a solar cell, photons from sunlight need to interact with and excite electrons, causing the electrons to leave their atomic cores and generating an electrical current, Guo said. Ideally, the solar cell would use materials that are weak to pull the excited electrons back to the atomic cores and stop the electrical current.
The researchers showed that such a recombination could be substantially prevented by combining a perovskite material with either a layer of metal or a metamaterial substrate consisting of alternating layers of silver, a noble metal, and aluminum oxide, a dielectric.
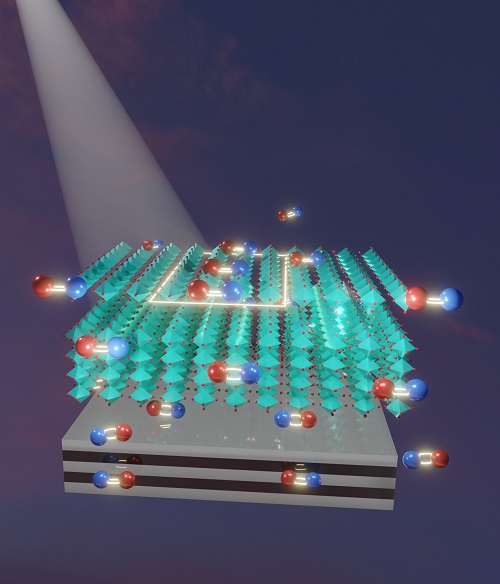
This illustration shows the interaction between a perovskite material (cyan) and a substrate of metal-dielectric material. The red and blue pairings are electron-hole pairs. Mirror images reflected from the substrate reduce the ability of excited electrons in the perovskite to recombine with their atomic cores, increasing the efficiency of the perovskite to harvest solar light. Courtesy of Chloe Zhang.
“A piece of metal can do just as much work as complex chemical engineering in a wet lab,” Guo said.
The result was a significant reduction of electron recombination. In effect, the metal layer served as a mirror that created reversed images of electron-hole pairs, weakening the ability of the electrons to recombine with the holes.
According to the researchers, suppressing the recombination processes in lead-halide perovskites without the use of chemical treatments is itself a challenge.
Using a simple detector, the researchers observed the resulting 250% increase in efficiency of light conversion.
Several challenges remain before perovskites become practical for their applications. Their tendency to degrade quickly is among the hurdles. Researchers are working to find new, more stable perovskite materials.
“As new perovskites emerge, we can then use our physics-based method to further enhance their performance,” Guo said.
As for the result, in addition to implications for solar cells, the researchers said the approach provides novel means to enhance efficiency of perovskite-based optoelectronic and photonic devices.
The Bill and Melinda Gates Foundation, the Army Research Office, and the National Science Foundation supported the research, which was published in Nature Photonics (www.doi.org/10.1038/s41566-022-01151-3).