Pure light is good for many bioimaging studies, but it has insurmountable limitations
when it comes to scanning beneath the surface of the skin. Light in the near-IR
range of about 2 to 3 µm can penetrate tissue up to only 0.1 to 1 mm, far too shallow
to search for anything more than subcutaneous evidence of healthy activity or disease.
Wavelengths between 650 and 1300 nm can reach 1 to 2 mm before light scattering
within the tissue reduces image resolution too much to be useful.
Sound waves, however, aren’t scattered nearly as much by
a tissue’s cellular and water content as light is, leading some researchers
to pursue ultrasound techniques to image living organisms. But where ultrasound
has the desired penetrability, it does not offer the high resolution that purely
light-based imaging does.
Unsurprisingly, then, the two technologies came together. Or,
rather, they were pushed.
About 20 years ago, a scientist with ultrasound experience was
looking at novel piezo-based transducers that might provide the higher frequencies
and smaller scale he needed for better images and easier use. That search led Matthew
O’Donnell, now dean of the college of engineering at the University of Washington
in Seattle, to investigate photoacoustic techniques, in which light and sound work
together. He hasn’t turned back, and many others have joined him.
 In a typical photoacoustic setup, pulses of laser light shine
on a target, which can be a photoreactive agent, a glistening nanoparticle or
a natural substance such as hemoglobin. As the target absorbs the laser’s
energy, part of its response is to emit an ultrasonic noise. The sound waves thus
emitted are, in turn, detected by a nearby array of piezoelectric or fiber optic
sensors and converted into images that can be read by the device’s operator.
In a typical photoacoustic setup, pulses of laser light shine
on a target, which can be a photoreactive agent, a glistening nanoparticle or
a natural substance such as hemoglobin. As the target absorbs the laser’s
energy, part of its response is to emit an ultrasonic noise. The sound waves thus
emitted are, in turn, detected by a nearby array of piezoelectric or fiber optic
sensors and converted into images that can be read by the device’s operator.
With fairly high resolution and an ability to plumb relatively
large depths into tissue – especially compared to infrared imaging –
photoacoustic technology is useful for a number of imaging purposes. The technique
is used to search for tumors and lesions, to investigate blood vessels and other
parts of the vasculature, and to examine targets from whole animals down to individual
cells.
Using photoacoustics to image blood vessels is particularly useful
for studying the way cancer tumors feed and grow. And it is a useful tool for observing
hemodynamics because it can differentiate between the total hemoglobin in blood
and the hemoglobin that is saturated with oxygen.
Photoacoustics is also useful during patient therapy for procedures
that include measuring temperature, and helping with placement of tubes and needles
for drug delivery and biopsies, and with radioactive seeds for brachytherapy. Nonetheless,
its main purpose is diagnostic and therapeutic imaging.
Where optical imaging and ultrasound imaging once were separated
into two very distinct camps, according to O’Donnell, there has come a change
in everyone’s approach.
“We are starting to see the integration of optics and ultrasound
thinking,” he said. And new photoacoustic tools someday “will become
the core part of everyday medical treatment.”
Needles and haystacks
Cancer metastases are a major cause of patient mortality. Tumors
metastasize when individual cells break away from an active site and float down
the bloodstream, adhere to another site and begin to generate a new tumor and supporting
blood vessels. Traditional imaging techniques, such as computed tomography, can
find these new tumors when they’ve grown to a few millimeters, but by then
it is likely that the patient is well into the final stages of disease. Finding
the breakaway tumor cells while they are still midstream would be a powerful tool
to pinpoint – and perhaps challenge – metastasis. Several research groups
have developed techniques for getting the job done.
John Viator, associate professor of bioengineering and dermatology
at the University of Missouri in Columbia, is developing a photoacoustic flow cytometry
method that can look for single cancer cells in blood samples. In a basic setup,
blood flowing past a photoacoustic sensor is struck by laser light. Certain constituents
of the blood, such as melanin, hemoglobin and tumor cells, emit a characteristic
sound wave as a response to the light.
Viator’s group is focused on using the technique to look
for circulating melanoma cells and has tested its photoacoustic system to train
nanosecond-scale laser pulses on melanoma-laden white blood cells that have been
extracted from whole blood. The irradiation of these samples causes a distinct high-frequency
sonic response from the melanoma cells hidden among the white blood cells, according
to the researchers. The group has since verified that even individual melanoma cells
are detectable with the technique.
“Photoacoustics combines the selectivity of optical targeting
with the sensitivity of ultrasonic signals,” Viator said.
Finding individual cancer cells, however, is not enough; you also
must remove them before they start a new tumor.
Vladimir P. Zharov of the University of Arkansas at Little Rock
has been performing pioneering work in photoacoustic flow cytometry advances during
the past decade. Working in a broad range of applications, Zharov and his colleagues
are studying the effects of labeling target cells with gold-coated carbon nanotubes,
quantum dots or magnetic nanoparticles; exploring multicolor photoacoustic imaging;
and testing laser systems that will increase the imaging speed and sensitivity of
single-cell detection.
In studies of sentinel lymph nodes – often the first landing
spot for stray cancer cells – Zharov’s group used a fiber-based laser
system to detect and destroy melanoma cells. The system it used for this and most
subsequent projects comprises an Olympus microscope; a tunable optical parametric
oscillator made by Lotis Ltd. of Minsk, Belarus; a 905-nm diode laser manufactured
by Frankfurt Laser Co. of Friedrichsdorf, Germany; and an ultrasound transducer
made by Imasonic Inc. of Besançon, France.
The investigators used gold-coated carbon nanotubes as a contrast
agent within the lymph system, gaining a second color, which aided identification
of the lymph nodes and vessels by employing magnetic nanoparticles composed of Fe2O3
cores coated with polyethylene glycol. Laser pulses of 639 nm proved to be the optimal
setting to reveal the magnetic particles, whereas 850 nm provided maximum absorption
with the gold nanotubes.
Functionalizing the gold nanotubes with folate allowed the researchers
to track breast cancer and melanoma cells with the lymph system, in separate attempts.
Increasing the laser pulse energy from a normal 20 mJ/cm2 to 100 mJ/cm2 enabled
the team to not only locate melanoma cells, but also to destroy them. At that energy
level, microbubbles form, surrounding the gold nanotubes or the cells themselves;
the heated bubbles disintegrate any nearby cancer cells.
“Since photoacoustics can target hemoglobin in blood vessels,
it is possible to reconstruct the vasculature and microvasculature,” said
the University of Missouri’s Viator. “It may be possible to use this
to identify cancerous tumors, which are known to be hypervascular.”
Getting a read on retinas
For some researchers, nowhere is the vasculature more interesting
than inside the eye. Acquiring high-resolution 3-D images of the retina’s
microvasculature, in particular, or of the retinal pigment epithelium (RPE), could
provide important insights into a number of diseases, including diabetic retinopathy
and age-related macular degeneration.
Hao F. Zhang, an assistant professor at the University of Wisconsin-Milwaukee,
believes that ocular imaging using photo-acoustic techniques has the greatest potential
to be adopted into clinics. He and his colleagues have worked in photoacoustic microscopy
for several years and have combined the technique with other optical methods, such
as confocal and fluorescence microscopy and optical coherence tomography.

Figure 1. Researchers at the University of Wisconsin-Milwaukee combine spectral-domain
OCT with photoacoustic optical microscopy. Schematics show the system layout (left)
and the path of the optical beam to the retina and the position of the slim ultrasonic
transducer (right). The two imaging subsystems are synchronized by the photoacoustic
optical microscopy laser pulses detected by photodiode #1. SLD = superluminescent
diode; UT = ultrasonic transducer; Pd = photodiode; FOV = field of view; PC = polarization
controller. Reused with permission of the Optical Society of America.


Figure 2. With the University of Wisconsin’s system, OCT and
photoacoustic optical microscopy images are acquired simultaneously in vivo. Compare
a photoacoustic optical microscopy B-scan image in pseudocolors (a) with an OCT
B-scan image (b). A maximum amplitude projection image of the photoacoustic optical
microscopy data set is shown in (c). Scale bar = 100 μm. HA = hyaloid artery
(remnant); RPE = retinal pigment epithelium. Reused with permission of the Optical
Society of America.
Hemoglobin and melanin strongly absorb light, providing contrast
against the rest of blood’s components and thus providing a good basis for
both functional and anatomic imaging of the retina’s vasculature and the RPE,
according to Zhang’s group.
“The unique optical absorption contrast mechanism,”
Zhang said of photoacoustic microscopy, “is not currently available elsewhere.”
To the clinic
Photoacoustic technologies are just beginning to be adapted into
clinical settings, according to the University of Missouri’s John Viator and
other researchers. They are too new to have matured into the role.
“Much of the early work in biomedical photoacoustics was
done in the mid to late 1990s, and much of the technology is now becoming mature
enough to be used clinically,” Viator said. “I predict an increasingly
active industry using photoacoustics in the next decade.”
To some, it’s a matter of presenting a technology with which
clinicians already are familiar. According to Wiendelt Steenbergen, a professor
at the University of Twente in Enschede, the Netherlands, new photoacoustic technology
must be integrated with already established ultrasound imaging.
Among the topics with which Steenbergen and his associates are
occupied is photoacoustic mammography. Traditional x-ray mammography is well known
to have problems with ionizing radiation, yet it remains the gold standard for cancer
detection. Magnetic resonance imaging suffers from low specificity and high cost;
ultrasound imaging alone offers too-low sensitivity; and infrared imaging does not
have the penetrating power to reach most subcutaneous tumors because of light scattering.
To address the problems with all of these techniques, Steenbergen’s
group developed a system a few years ago that it has dubbed the “Twente photoacoustic
mammoscope.” The device uses a beam from a Q-switched Nd:YAG laser made by
Paris-based Quantel operating at 1064 nm to acoustically excite breast tissue. The
team members used a lab-built ultrasound detector with 590 elements to pick up the
resulting sonic emissions. Figure 3 shows some resulting images.
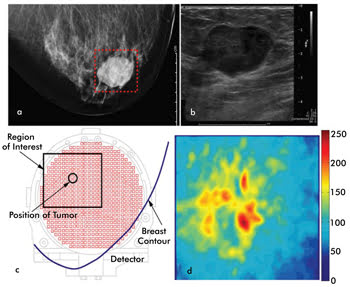
Figure 3. Shown are images acquired by Wiendelt Steenbergen’s group at the University
of Twente via craniocaudal x-ray mammography (a) and via ultrasound (b), with each
showing a large tumor with well-defined margins. The area judged to be the region
of interest is outlined in the x-ray image. The contour of the breast is shown under
compression in the researcher’s photoacoustic mammogram system, with the region
of interest and possible location of a tumor indicated (c). The maximum intensity
projections of the 3-D reconstructed photoacoustic data are shown in (d). The ring-shaped
region of high intensity likely indicates the tumor rim, where blood vessels are
plentiful. Reused with permission of IEEE Journal of Selected Topics in Quantum
Electronics.
“Photoacoustics enables imaging optical absorption at a
high resolution, even at tissue depths where optical scattering prevents high-resolution
optical imaging,” Steenbergen said. To achieve significant depth, however,
each laser pulse must be at least 50 µJ, which requires beam expansion so that patients’
skin isn’t at risk.
The system is undergoing clinical trials, and Steenbergen’s
team has also turned toward speeding up the mammoscope’s imaging ability,
gaining contrast enhancement via gold nanorods and improving the detector’s
angular field of view. Steenbergen also has identified several general areas where
photoacoustic technology must be improved, including quantifiability, a better selection
of light sources with sufficient pulse energy to gain depth of penetration into
tissue, and a better broadband ultrasound array detector with sufficient sensitivity.
A sound technology
According to the University of Arkansas’ Vladimir Zharov,
photoacoustics research is being driven by improvements in “optical resolution
down to the diffraction limit (200 to 250 nm), temporal resolution up to 10 to 100
µs for high-speed imaging and detection of dynamic events (e.g., moving cells),
and multispectral capability for real-time multicolor cytometry.”
One possible path to resolution improvement is to update the transducer
from its piezo-based origins. Günther Paltauf of Karl Franzens University of
Graz in Austria and his colleagues have eschewed traditional acoustic detection
for optical detection of the emitted sound waves. Although it remains the standard
method for sound wave detection, piezo-based transducers lose sensitivity as their
scan heads shrink, and smaller devices will be necessary to spread into more clinical
use.
“We are specializing in developing detection methods for
photoacoustic imaging and related image reconstruction algorithms,” he said.
“A strong focus is on optical detection of ultrasound, which has several advantages
compared to conventional piezoelectric detection.”
The technique developed by Paltauf’s team is based on a
laser beam that is focused on the target but is left to propagate freely in a coupling
liquid that surrounds the target. When a second beam triggers the photoacoustic
reaction in the target, the emitted sound waves cause small phase changes in the
freely propagating beam. These minute changes in phase are detected by a Mach-Zehnder
interferometer. The team has used this technique to create high-resolution 3-D images
of mouse hearts, human hairs and other structures (Figure 4).

Figure 4. A group led by Günther Paltauf of Karl Franzens University of Graz used 3-D
photoacoustic imaging to study mouse hearts. Shown here are four sections of one
such image. RV = right ventricle; LV = left ventricle; LA = left atrium; MV = mitral
valve and PM = papillary muscles. Reused with permission of the Journal of Biomedical
Optics.
According to Paltauf, however, there remain limitations to purely
photoacoustic techniques.
“The imaging information is somewhat limited because only
optical absorption contrast can be seen,” he said. “It is therefore
desirable to combine photoacoustic with other purely ultrasonic or purely optical
methods to gain additional, complementary information.”
Whether photoacoustics becomes part of a multimodal imaging package
or continues with breakthroughs by itself, there is no doubt that it has momentum.
“Photoacoustics has to compete with several already accepted
and mature imaging modalities, such as MRI, ultrasound imaging and CT scans,”
Steenbergen said. “Compared to these, photoacoustics has unique features [but]
still has to reach a higher stage of sophistication to find acceptance in clinical
research.”
Zharov foresees a time when photo-acoustic technology will become
a mainstay, with noninvasive, rapid examination of nearly all of the body’s
entire three to five liters of blood undergoing examination for single cancer cells,
pathogens or other intruders with a sensitivity that is not possible with other
technologies.