
Smartphone-Based NIRS Imaging Classifies Wounds
By measuring tissue oxygenation via NIR spectroscopy, clinicians could triage diabetic foot ulcers and determine whether immediate intervention is needed.
By Anuradha Godavarty, Daniela Leizaola, and Kacie Kaile
Diabetes mellitus affects >32.2 million people in the U.S. and an additional 12.5% of the U.S. population is prediabetic1. One in three people with diabetes is affected by diabetic foot ulcers (DFUs) during their lifetime2. If this condition is undiagnosed or untreated, DFUs become infected and require amputation, thus reducing the lifespan of these patients. In a clinical setting, visual assessment is the gold standard, but current smartphone technologies for wound care are limited to 2D/3D wound image analysis for size or depth. A smartphone-based near-infrared spectroscopic (NIRS) imaging approach, or smartphone oxygenation tool, was recently developed to obtain visual and physiological measurements of tissue oxygenation in wounds — a vital factor for healing.
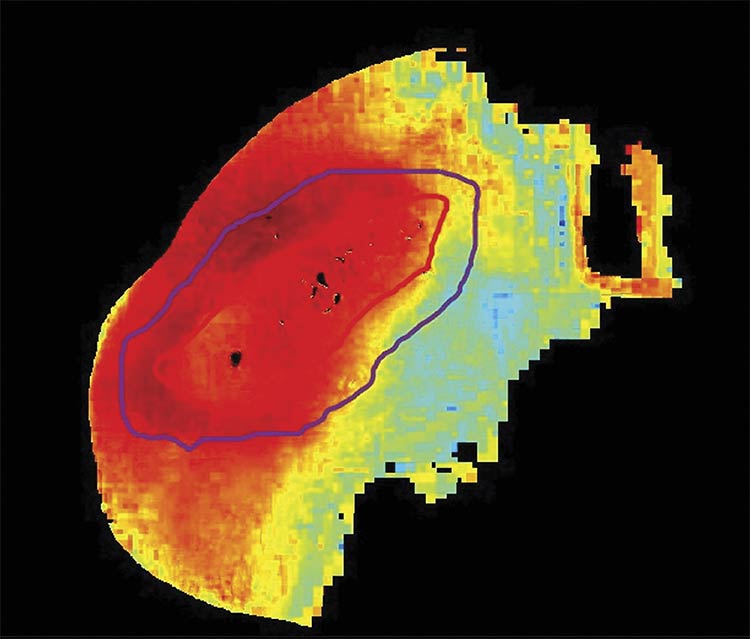
An oxygenation saturation map (StO2) of a healing post-debrided diabetic foot ulcer. Courtesy of Florida International University.
Wound care management and early prediction or improved prognosis of DFUs during the treatment process are vital to enhance the lifespan and quality of life of patients. Due to the recent pandemic, challenges to accessibility to wound care by low-income populations, the elderly, and patients living in remote areas have led to a paradigm shift from clinical care to community care for wound treatment. Remote patient monitoring and telemedicine are crucial in wound care management to serve these populations.
Smartphone-based wound care
The gold-standard clinical assessment of DFUs includes a visual assessment of wound coloration, smell, warmth, and pedal pulses, along with manual measurement of the wound area (length and breadth). A 50% reduction in wound size within four weeks is an indicator of healing, according to the Wound Healing Society.
Smartphone-based technologies have been used in wound care management in various ways: wound-related apps for clinicians to assess wound size (2D and 3D with depth) and area changes over weeks from digital images of the wound; and wound care apps that remind patients of their daily dos and don’ts for caring for their wounds. While these apps digitize the manual visual assessment, they do not determine the underlying physiology that contributes to the healing or nonhealing nature of DFUs.
The Optical Imaging Laboratory at Florida International University (FIU) developed a smartphone-based NIRS optical imaging device, called SPOT, which is an oxygenation tool that can assess tissue oxygenation changes in and around wounds3,4. Assessing the oxygen bound to the blood (or oxygenated blood) can determine how much oxygen enrichment is reaching the wound site to promote its healing. NIRS technology measures this oxygenated blood in terms of oxy-, deoxy-, total hemoglobin, or oxygen saturation (or tissue oxygenation).
SPOT uses an Android-based smartphone, an add-on optical module, and a custom-developed app for automated data acquisition and analysis. Multiple multiwavelength LEDs, between 690 and 830 nm, are controlled by an LED driver in the add-on module, and diffused NIR light at each wavelength is multiplexed to illuminate large areas during imaging studies3,4. The diffuse reflectance signals from the imaging surface are detected by the smartphone’s NIR-sensitive camera after it passes through a longpass filter. The LED driver is powered by the smartphone and Bluetooth-controlled by the custom SPOT App, developed to automate multiwavelength NIR illumination and detection. The use of the SPOT device on a foot model with DFUs is shown in Figure 1.

Figure 1. The smartphone oxygenation tool (SPOT) hardware with an optical module attached to the smartphone (left). The smartphone display showing camera controls and real-time diffuse reflectance signals (right). Courtesy of Florida International University.
Tissue oxygenation analysis
2D diffuse reflectance images obtained at 690 nm and 830 nm were used with the modified Beer-Lambert’s Law to determine the effective oxy- (HbO), and deoxy- (HbR) maps. These effective maps were in turn used to obtain the effective total hemoglobin (HbT) and oxygen saturation (StO2).
The SPOT device has been validated via phantom and in vivo studies. During phantom studies, the device was used to image a 10- × 10- × 10-cubed-cm tissue phantom of known absorption and scattering properties. The detected 2D diffuse reflectance maps from the phantom surface were compared to simulated diffuse reflectance signals obtained via Monte Carlo light propagation models — that is, random sampling to determine probability. The imaging distance between the phantom and SPOT’s detector was optimized between 8 and 10 cm to maximize the signal-to-noise ratio to >20 dB. A 2D correlation analysis of the experimental and simulated diffuse reflectance maps was carried out. The correlation was >94% and 96% for the diffuse reflectance maps obtained at 690 nm and 830 nm, respectively, between SPOT and Monte Carlo simulations5, thus demonstrating phantom validation of the SPOT device.
SPOT’s ability to image DFUs for tissue oxygenation changes was validated against a commercial NIRS device via clinical imaging studies in collaboration with Robert Scott Kirsner and the team at the University of Miami Health System. In this Institutional Review Board (IRB)-approved study, 14 DFU cases were imaged using SPOT and the commercial NIRS device via noncontact imaging5. Tissue oxygenation-based parameters (in terms of effective HbO, HbR, HbT, and StO2) were reconstructed from diffuse reflectance signals obtained from the SPOT device.
The oxygen saturation maps from SPOT were compared to those obtained from the commercial NIRS device. Regions of darker skin colors were eliminated from the oxygen saturation maps of the commercial device. Hence, only the equivalent regions from SPOT were compared via 2D correlation analysis. A median correlation of 75% and a range of 57% to 90% correlation was observed between the oxygen saturation maps obtained using the SPOT and commercial device and across the 14 DFU cases5.
Clinical applications of SPOT
Following the validation of SPOT for obtaining 2D tissue oxygenation maps in vivo, SPOT has been applied to various clinical applications related to DFUs. The applications include screening or triaging patients who need immediate clinical intervention, performing image-guided wound debridement to assess its effectiveness in improving flow to the wound site, and differentiating healing from nonhealing DFUs (Figure 2).
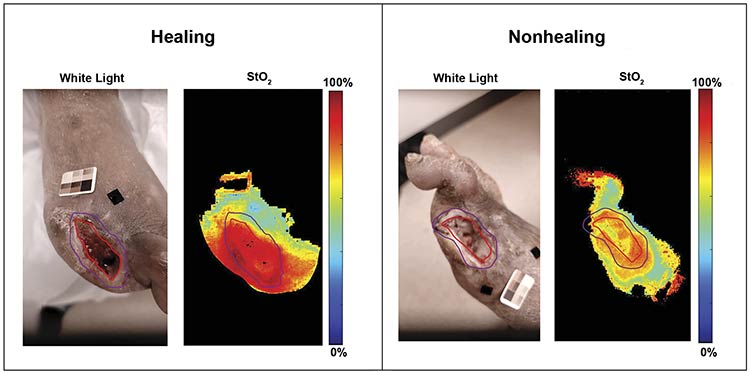
Figure 2. White light and oxygenation saturation maps (StO2) of healing and nonhealing post-debrided diabetic foot ulcer cases. The red and purple contour tracing is the clinician’s traced wound and peri-wound boundary, respectively.
Courtesy of Florida International University.
In a clinical study conducted in collaboration with Viswanathan Mohan and the team at Dr. Mohan’s Diabetes Specialties Centre in Chennai, India, the researchers focused on differentiating high-risk from low-risk DFUs. High-risk DFUs were defined as those requiring immediate clinical intervention, either as outpatient or inpatient surgical debridement. Low-risk DFUs were those that did not require immediate clinical intervention because the chronic DFUs were stable. Tissue oxygenation maps (effective oxy-, deoxy-, total hemoglobin, and oxygen saturation) were obtained using the SPOT device across three high-risk and 16 low-risk DFU cases in this IRB-approved study5.
When comparing the tissue oxygenation distribution between the wound (region 1) and peri-wound areas (regions 2 and 3) in Figure 3, homogeneity was observed in the low-risk DFU case, compared to a heterogeneous distribution in the high-risk DFU case. A wound- and peri-wound-based contrast in each tissue oxygenation parameter was analyzed to develop a tissue oxygenation-based metric that differentiates high-risk from low-risk DFUs. The researchers determined that the total hemoglobin-based contrast differentiated the two risk types of DFUs with 100% sensitivity and 84% specificity5.
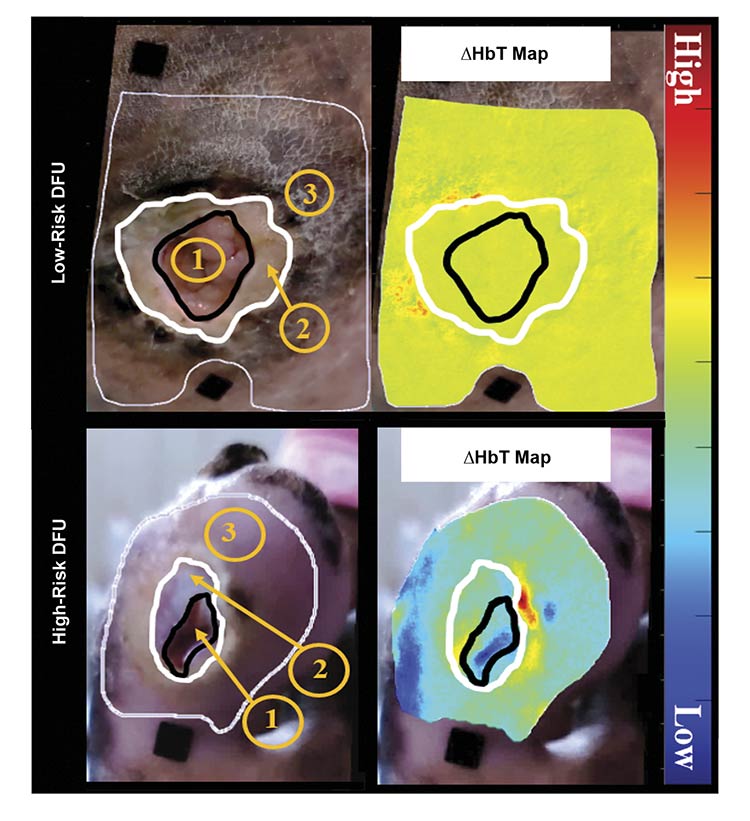
Figure 3. White light and effective total hemoglobin (HbT) maps of low-risk and high-risk diabetic foot ulcer (DFU) cases. Region 1 is the wound, region 2 is the nonepidermal peri-wound region, and region 3 is the area where tissue oxygenation analysis was performed to encompass the immediate surrounding epidermal peri-wound tissue. Courtesy of Florida International University.
Image-guided debridement
Scalpel debridement aims to promote the healing process by reintroducing a wound site to the inflammatory stage. The procedure involves removing tissue using a sharp knife until the tissue appears pinkish or bleeding is observed. This is typically assessed visually, and the approach is subjective. SPOT was used to evaluate tissue oxygenation changes in response to the debridement procedure in a clinical study conducted in collaboration with David Armstrong and his team from the University of Southern California at Clemente Clinical Research and White Memorial Group in Los Angeles. In this IRB-approved study, DFU subjects were imaged using SPOT before and immediately after the debridement procedure for tissue oxygenation changes.
A total of 16 subjects were recruited across one to four weeks during this study. In a preliminary analysis, there was an increase in effective oxygen saturation in the post-debrided region after the procedure was conducted6. The degree of debridement was found to be relevant to the extent of change in the effective oxygen saturation in the debrided region. This demonstrates that SPOT could serve as an image-guided debridement tool during outpatient clinical treatment of DFUs.
Healing versus nonhealing
As stated at the beginning of this article, according to the Wound Healing Society, a 50% reduction in wound size within four weeks is considered indicative of a healing DFU. In collaboration with Armstrong and his team, a clinician’s assessment of the healing or nonhealing trajectory was used as a ground truth to determine if the tissue oxygenation-based parameters could assess whether the DFU was considered healing or nonhealing. The wound and peri-wound areas were traced by the clinician. A wound-to-peri-wound contrast of each tissue oxygenation-based parameter was determined using 21 cases across 9 subjects.
Preliminary studies demonstrated that the total hemoglobin and oxygen saturation-based contrast could classify the wound as healing or nonhealing with an accuracy of ~80%6. Ongoing efforts are focused on extensive statistical analysis and developing a regression model (in collaboration with Wensong Wu, a biostatistics expert at FIU) to predict healing potential using the weekly tissue oxygenation data from DFUs.
SPOT in real-world translation
SPOT captures diffuse reflectance data from the skin’s surface. But skin pigmentation varies across populations due to the concentration of melanin in the topmost layer of the skin, called the epidermis. Melanin is a large absorber within the NIR range and the absorption due to melanin can mask the actual tissue oxygenation from SPOT. Melanin concentration is positively correlated with darker skin color. Skin color can be objectively classified using the Fitzpatrick Skin Type (FST) scale ranging from I to VI and correlated to the increase in melanin concentration with an increase in FST.
Monte-Carlo light-propagation models were used to perform simulated phantom studies of varying skin colors (or FST I-VI with varying melanin concentrations), and a melanin correction factor was developed. The error in the reconstructed HbO and HbR due to melanin ranged from 5% to 55% across the FST I-VI. Upon implementing the melanin correction, the error in HbO and HbR reduced to ≤5% across all the FSTs7. Thus, accounting for melanin — potentially with the aid of machine learning — could improve the applicability of the SPOT device across various skin colors, that is, across various racial and ethnic groups.
While tissues are usually curved, the detected image of the tissue surface is a projection onto the focus plane during imaging studies. In the case of NIR optical imaging, the curvature of the tissue, especially the curvature in a foot, is not an accurate representation of the underlying physiology, because curvature can also affect the detected diffuse reflectance signals. Hence, there is a need to assess the extent of tissue curvature, its impact on the diffuse reflectance signals, and the tissue oxygenation maps to develop a curvature correction factor. Ongoing studies are focused on validating the developed curvature correction factors via Monte-Carlo-based simulation studies on foot-mimicking phantom models and experimental curved phantoms8.
Future work
To date, the smartphone-based noncontact NIRS imaging device SPOT has been developed to measure tissue oxygenation changes in DFUs. Ongoing efforts aim to improve image analysis models that account for the effects of melanin during tissue oxygenation measurements, as well as the effects of tissue curvature, especially in post-amputation DFU cases.
In a parallel effort, a multimodal smartphone-based optical-thermal imaging device is currently being developed9, integrating NIRS with a thermal sensor to allow a comprehensive assessment of wound physiology and evaluation of the healing potential, enabling prediction of the onset of inflammation/infection and ischemia in DFUs. The integration of thermal imaging is particularly valuable, as it can detect localized temperature differences in the foot in response to infection/ischemia. Since both infection and ischemia are key contributors to amputations in DFUs, monitoring these parameters is essential.
In combination, these physiological markers can help clinicians to stratify patients based on wound severity and risk, enabling earlier, more targeted interventions and ultimately improving clinical outcomes in DFU management. By combining real-time measurements of tissue oxygenation using NIRS with localized temperature changes from thermal imaging, this multimodal SPOT device will offer improved, objective, and comprehensive DFU care management.
Meet the authors
Anuradha Godavarty is a professor of biomedical engineering at Florida International University with more than 25 years of experience in the field. Her research focuses on the development of hand-held and smartphone-based near-infrared spectroscopy (NIRS) and multimodal imaging devices, with an emphasis on translating these technologies into clinical applications; email: godavart@fiu.edu.
Kacie Kaile earned her Ph.D. in Biomedical Engineering at Florida International University under the mentorship of Anuradha Godavarty. She is one of the co-inventors of the SPOT imaging device. Kaile is currently based in the Florida Keys, where she is actively launching two startup ventures; email: kkail001@fiu.edu.
Daniela Leizaola is a Ph.D. candidate in Biomedical Engineering at Florida International University, working under the guidance of Anuradha Godavarty. Her research investigates the impact of skin color on tissue oxygenation maps generated by the SPOT device. Leizaola was recently awarded the FIU Dissertation Year Fellowship to support the final year of her doctoral studies; email: dleiz001@fiu.edu.
Acknowledgments
This work was supported by several funding sources: FIU Dissertation Year Fellowship (to Kacie Kaile), NIH-NIBIB R01-EB0033413, FIU-BME Coulter Funds, and NSF I-Corps grant. The authors would like to thank the entire research team at the Optical Imaging Laboratory for contributing to this research. The authors would also like to thank the staff, residents, and clinicians at the respective clinical sites where imaging studies on DFUs were carried out using SPOT for their assistance and support.
References
1. International Diabetes Federation, “IDF Diabetes Atlas, 10th edn. 2021, IDF,” International Diabetes Federation, Brussels, Belgium, 2021.
2. K. McDermott et al. (2023). Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care, Vol. 46, No. 1, pp. 209-221.
3. K. Kaile and A. Godavarty. (2019). Development and validation of a smartphone-based near-infrared optical imaging device to measure physiological changes in-vivo. Micromachines (Basel), Vol. 10, No. 3.
4. K. Kaile et al. (2021). Development of a smartphone-based optical device to measure hemoglobin concentration changes for remote monitoring of wounds. Biosensors (Basel), Vol. 11, No. 6.
5. K. Kaile (2023). A smartphone-based near-infrared imaging device to obtain tissue oxygenation maps in diabetic foot ulcers. Doctoral thesis. Florida International University.
6. D. Leizaola et al. (January 2025). Objective assessment of clinical debridement and healing status of diabetic foot ulcers using a smartphone-based NIRS device. In Optical Diagnostics and Sensing XXV: Toward Point-of-Care Diagnostics. Proc SPIE, San Francisco, Calif.
7. D. Leizaola et al. (January 2025). Development and validation of a melanin correction factor for a smartphone-based NIRS optical imaging device. In Optical Diagnostics and Sensing XXV: Toward Point-of-Care Diagnostics. Proc SPIE, San Francisco, Calif.
8. H. Shakhar Roy et al. (January 2025). Curvature correction in near-infrared spectroscopy for diabetic foot ulcers: a phantom study. In Optical Diagnostics and Sensing XXV: Toward Point-of-Care Diagnostics. Proc SPIE, San Francisco, Calif.
9. F.S. Chiwo Gonzalez et al. (January 2025). Implementation of an optical-thermal imaging mobile scanner for diabetic foot ulcers monitoring. In Multimodal Biomedical Imaging XX. Proc SPIE, San Francisco, Calif.
Published: September 2025