SAN DIEGO, Feb. 23, 2009 – Nanoscale materials are the topic of much debate because they often are considered too poisonous for use in humans.
Now a team of scientists has created minuscule flakes of silicon that glow brightly, last long enough to slowly release cancer drugs, then break down into harmless by-products.

New silicon nanoparticles illuminate tissues without harm. Images courtesy of Luo Gu, University of California, San Diego.
“It is the first luminescent nanoparticle that was purposely designed to minimize toxic side effects,”
said Michael Sailor, a chemistry professor at the University of California, San Diego, who led the study. “This new design meets a growing need for nontoxic alternatives that have a chance to make it into the clinic to treat human patients.”
The particles inherently glow, a useful property that is most commonly achieved by including toxic organic chemicals or tiny structures called quantum dots, which can leave potentially harmful heavy metals in their wake.
When the researchers tested their safer nanoparticles in mice, they saw tumors glow for several hours, then dim as the particles broke down. Levels dropped noticeably in a week and were undetectable after four weeks.
This is the first study to image tumors and organs using biodegradable silicon nanoparticles in live animals, the authors say.
The particles begin as thin wafers made porous with an electrical current, then are smashed to bits with ultrasound. Additional treatment alters the physical structure of the flakes to make them glow red when illuminated with ultraviolet light.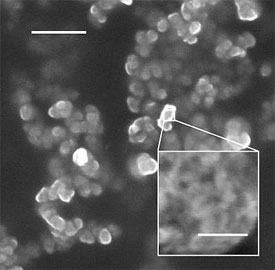
This is a microscopic view of silicon flakes designed to deliver drugs and then dissolve.
Luminescent particles can reveal tumors too tiny to detect by other means or can allow a surgeon to ensure that a cancerous growth has been removed totally.
The nanoparticles also could help deliver drugs safely, according to the researchers. The cancer drug doxorubicin will stick to the pores and slowly escape as the silicon dissolves.
“The goal is to use the nanoparticles to chaperone the drug directly to the tumor, to release it into the tumor rather than other parts of the body,” Sailor said.
Targeted delivery would allow doctors to use smaller doses of the drug. At doses high enough to be effective, when delivered to the whole body, doxorubicin often has toxic side effects.
At about 100 nm, these particles are bigger than many designed to deliver drugs, which can be just a few nanometers across – a thousand times smaller than the diameter of a human hair.
Their larger size contributes to both their effectiveness and their safety. Large particles can hold more of a drug, yet they self-destruct, and the remnants can be filtered away by the kidneys.
Close examination of vulnerable organs such as liver, spleen and kidney, which help to remove toxins, revealed no lasting changes in mice treated with the new nanoparticles.
Graduate students Ji-Ho Park and Luo Gu in Sailor’s lab; Sangeeta Bhatia, bioengineering professor at MIT in Cambridge, and graduate student Geoffrey von Malzahn in Bhatia’s lab; and Erkki Ruoslahti, professor at the University of California, Santa Barbara, all contributed to this work.
The research was funded by the National Cancer Institute and the National Science Foundation.
For more information, visit: www.ucsd.edu