Optically pumped semiconductor laser (OPSL) technology dominates some of the leading applications for continuous wave (CW) and modulatable laser light, including those used for cytometry, sequencing, fluorescence microscopy and ophthalmological photocoagulation. OPSLs have rapidly transitioned from next-generation CW laser technology status to a dominant force within the life sciences. With more than 100,000 systems operating in the field, OPSL’s unique combination of advantages have made them highly successful tools.
DR. MATTHIAS SCHULZE, COHERENT INC.
In an OPSL, laser diodes are used to pump a thin semiconductor chip, thus enabling an incredible level of design flexibility in terms of power and wavelength scalability. The quantum well structure of the gain chip determines the OPSL’s output wavelength, which can be set anywhere along a wide range of the near-infrared spectrum. Efficient intracavity doubling (or tripling) then converts this to either a visible or UV output wavelength. As a result, an OPSL can fill in the wavelength gaps from the spectrum produced by laser diode modules.
OPSLs and laser diodes often are complementary for some low-power (i.e., tens-of-milliwatts) applications that require multiple wavelengths — the two technologies are often packaged in commercial plug-and-play products with an identical electrical, mechanical and optical format. Additionally, OPSLs can be directly and digitally modulated, albeit not as fast as diodes, but fast enough for applications such as flow cytometry.
An OPSL, however, is the preferred choice within many applications because it offers better beam quality than a laser diode. An OPSL’s resonator also locks in the optical performance throughout the laser’s lifetime, which can be as great as tens of thousands of hours. In contrast, the beam profile and wavelength of a laser diode shifts as it ages. Additionally, a laser diode’s beam profile and pointing change if the output power is varied; these properties remain fixed in an OPSL via its resonator.
Power scalability is another inherent advantage of OPSL technology, as output can be scaled from milliwatts to in excess of tens of watts. Indeed, for applications needing hundreds of milliwatts or multiple watts of laser power, OPSLs dominate because their only existing solid-state competition is the diode-pumped solid-state laser (DPSS), which offers only a handful of fixed visible wavelengths. In addition, unlike DPSSs and other such lasers using laser crystals, OPSLs can deliver extremely low-noise visible or UV light output, because there is no thermal lensing in the laser cavity. Plus an OPSL’s power can be smoothly adjusted over a wide range (from 5 to 100 percent of its rated power) with no effect on beam pointing or transverse mode structure.
Key application trends for OPSLs
In recent years, flow cytometry applications have come to be dominated by OPSLs. Today, many instruments use four or more different laser wavelengths and 15 or more different detection channels — different wavelength-specific fluorescence channels and scatter at one or more fixed angles. As new fluorochromes (i.e., dyes and fluorescent proteins) are developed, they are well-supported by new laser wavelengths due to an OPSL’s scalability. Also, these lasers’ modulation capabilities and stable beam output characteristic, particularly beam pointing and beam shape, are key advantages for both OEMs and end users.
Until recently, the primary cytometry-related applications were clinical hematology/immunology (e.g., counting blood cells, particularly CD4) and other somewhat diverse research applications, all of which were supported by large-frame, freestanding instruments. Today, well-established instrument makers and relative newcomers alike are pursuing the new area of economic benchtop instruments. One driver of this movement is the overarching trend of product miniaturization. The other driver comes from new applications, including those in biofuel research, epidemiology, oncology and stem cell research, as well as those within the pharmaceutical industry (such as faster screening and high-throughput discovery).
Instrument builders are supporting diverse applications with cost-effective benchtop instruments that feature a common platform but with a modular architecture that enables simple factory customization. Modularity typically includes the ability to use up to four different lasers and more than a dozen fluorescence and scatter detection channels, as well as different input modes, such as microwell plates for pharma and conventional flow tubes for blood analysis. Compact plug-and-play laser modules based on OPSL technology, such as Coherent’s OBIS product family, are proving to be very popular because they facilitate easy factory customization, as well as simplified instrument upgrade and field service.
A high level of efficiency and flexibility is due to the fact that every unit features identical optical, mechanical and electronic characteristics, irrespective of wavelength; the most commonly used wavelengths include 405, 488, 561 and 637 nm. Furthermore, the digital modulation capability of OPSL technology eliminates the cost and complexity of external modulators to support the timing sequences used in cytometry for multiwavelength excitation and detection. Just as important, the low noise and superior pointing stability of OPSLs support the sensitivity and speed demands of end-user applications, from research through to clinical use.
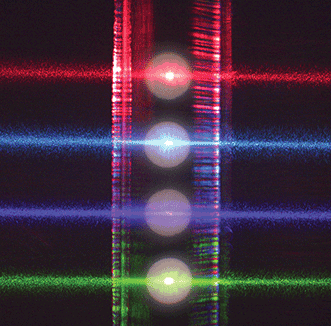
Figure 1. In many flow cytometers, multiple focused lasers intersect the flow cell. Courtesy of Thermo Fisher Scientific.
These lasers also support a key trend in large-frame instruments, namely the increasing use of UV laser excitation. The use of UV helps to avoid chemical intervention with fluorescent probes, used to excite endogenous fluorescence and/or to expand the bandwidth of multicolor analysis/acquisition. All living cells contain materials (e.g., NADH coenzyme and DNA molecules) that naturally will fluoresce when irradiated with UV light. For example, sperm can be sex sorted by simply comparing the amount of endogenous DNA fluorescence.
Noise issues (so-called “green noise”) associated with CW DPSS technologies have forced some instrument makers to resort to using mode-locked pseudo-CW DPSS systems as the UV source. Due to an OPSL’s upper-state lifetime being virtually zero, there is no such noise problem. In fact, true CW UV OPSLs are now available with powers as high as 250 mW. Such high power is ideal for use in such applications as the latest high-throughput cytometers that incorporate two parallel laser interaction cells.
Sequencing
The first human genome was read by multiple labs — each operating multiple sequencers — at a total cost of approximately $5 billion over the course of more than 10 years. Today, some sequencing instrumentation suppliers claim that their current technology offerings can read an entire human genome in one afternoon, and for a total cost somewhere between $100 and $1000. This tremendous change in cost has been made possible by instrument automation and by novel technologies that provide massive parallelism; next-generation sequencing (NGS) technology can simultaneously analyze as many as hundreds of thousands of strands of DNA. The NGS field has been dominated by Illumina Inc., which uses sequencing by synthesis, and Thermo Fisher Scientific Inc. (formerly Life Technologies), which uses sequencing by ligation (i.e., cutting). In both cases, sequencing is carried out in a flow cell excited with laser light. Each of the four nucleotides (adenine, cytosine, guanine, thymine) has a unique fluorescence emission profile. So each unique cluster (image location) flashes a wavelength pattern (color) that corresponds to the base being cut, in Thermo Fisher’s case, or next incorporated, in Illumina’s case.
Looking to the future, there are numerous, so-called “third-generation” sequencing technologies, in various stages of commercial development from many industry players. Although the technologies are very different and specific to each respective instrument maker, a common goal among them all is to achieve lower costs and faster throughput speeds. Laser-excited fluorescence is the detection method of choice in most of these technologies (see Figure 2), with some nonoptical methods competing. However, it’s not at all clear which, if any, of these sequencing technologies will dominate.


Figure 2. Massive parallelism is the key to high-throughput sequencing. For example, in one innovative third-generation method, up to 150,000 wells are patterned on a chip with a single strand trapped in each well. These are monitored simultaneously using laser excited fluorescence, in real time. As the bases are sequentially added in this sequencing by synthesis method, the fluorescence wavelength signature from each well indicates which of the four bases it is. Courtesy of Pacific BioSciences.
In terms of laser requirements, only a handful of wavelengths (e.g., 488, 514 and 532 nm, and red at approximately 640 or 660 nm) are required. That said, diverse choices are needed in every other laser parameter. This requires the widest-possible choice of standard lasers to support both R&D and, ultimately, volume production, especially with regard to output power.
If the sequencing technology involves many sites spread over large flow cells, or uses slides needing widefield illumination, the power requirement can increase to multiple watts. For applications where single strands are being sequenced and, therefore, signal is inherently low, power is especially critical because these applications usually feature massive parallelism. Conversely, some methods use only milliwatts of power in order to avoid damage. This diversity is best supported by more than one core technology. For example, diodes are sometimes the best choice for applications needing only low power and where cost is critical. OPSLs, though, always are preferred when beam quality and output power are paramount considerations.
Microscopy — confocal and superresolution
Since the first commercial models were developed at the argon ion legacy wavelength of 488 nm, OPSLs have been widely used in fluorescence microscopy, particularly confocal microscopy. Here, the beam quality and stability are key to producing high-resolution images with a superior signal-to-noise ratio. Moreover, the wavelength scalability of OPSLs has been a major benefit, as researchers have developed and deployed an increasingly broad palette of fluorophores. A recent example is the 588-nm wavelength, ideal for exciting the newer mFruit series of red fluorescent proteins, such as mPlum, mKate (TagFP63), mCherry and mRFP, as well as for popular labels such as Texas Red, Alexa Fluor 594, Katushka (TurboFP635), MitoTracker Red and CellTracker Red.
A dramatic trend in fluorescence microscopy has been the development of superresolution techniques to push optical imaging resolution past the classical diffraction limit to tens of nanometers and potentially even higher resolutions. These include methods based on deterministic photoswitching in space and time, as pioneered by Stefan Hell. Examples of this group include simulated emission depletion (STED) microscopy, ground state depletion (GSD) microscopy, reversible saturable optical fluorescence transition (RESOLFT) microscopy and saturated structured illumination microscopy (SIM). So-called stochastic methods achieve higher resolution by randomly switching single-molecule fluorescence on and off in space, as pioneered by Eric Betzig and William Moerner. Examples of the latter include direct stochastic optical reconstruction microscopy (dSTORM) and fluorescence photoactivation localization microscopy (FPALM); see Figure 3. Hell, Betzig and Moerner shared the 2014 Nobel Prize in chemistry for this work.
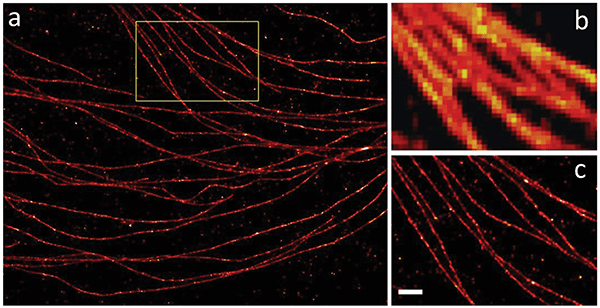
Figure 3. Stochastic microscopy imaging methods such as STORM deliver resolution levels far beyond the classical diffraction limit. Examples of STORM (a, c) and normal (b) resolutions are shown. Characterized by 40-nm resolution. Scale bar: 500 nm. Courtesy of professor Zhen-li Huang’s research team at Huazhong University of Science and Technology.
OPSLs are proving ideal for these methods for several reasons. First, higher laser power — up to a few watts — is required for optimal performance in several of these techniques. In the case of STED, for example, increasing the power of the laser constrains the fluorescence excitation to an ever smaller volume, thereby delivering higher resolution. In stochastic methods like STORM, it is essential to balance the density of fluorophore doping and the activation laser’s power in order to achieve an optimal density of single-point emitters in each frame. To support these applications with the widest choice in fluorophores, Coherent offers OPSLs at the multiwatt power level at several specific visible wavelengths.
Ophthalmology
Photocoagulation, used to treat the wet form of age-related macular degeneration (AMD), is arguably the single best application that highlights the benefits of OPSL technology’s level of wavelength scalability. Here, laser power is used to seal leaking blood vessels in the patient’s retina. The application needs several watts of power at a visible wavelength, allowing it to be delivered through the front of the eye without any damage to either the lens or aqueous humor. A few years ago, photocoagulation for AMD was dominated by lasers operating at 532 nm, as this was the most readily available wavelength offered by a DPSS laser.
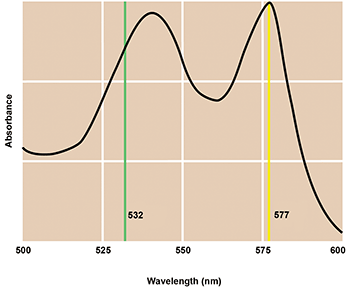
Figure 4. Coherent’s Genesis-557 was developed to improve laser photocoagulation outcomes for sufferers of certain forms of macular degeneration. The 577-nm output wavelength matches the absorption maximum of oxyhemoglobin and now dominates this important medical application. Courtesy of Coherent.
Tissue selectivity is the goal of photocoagulation — to seal the leaking blood vessel without causing any peripheral damage by the laser and with the absolute minimal amount of laser power in order to reduce any discomfort. The red color of blood is due primarily to oxyhemoglobin; 532 nm is near a weak absorption peak of oxyhemoglobin. To deliver potentially better outcomes, multiwatt OPSLs were developed exactly to match a stronger absorption peak at 577 nm. In addition to providing a more efficient wavelength match, it turned out that another OPSL characteristic — direct fast pulsing — also enabled much tighter control over the dosing than was possible with earlier DPSS iterations. As a result, the OPSL at 577 nm soon completely displaced the 532-nm DPSS; today, it’s the de facto gold standard for this critically important medical procedure.
Life sciences applications for CW lasers are very diverse; each application has its own specific laser requirements. OPSL technology’s uniquely broad wavelength and power scalability has proven be an excellent match for challenges in the field and have led to its ongoing market success.
Meet the author
Matthias Schulze, PhD, is the director of marketing for OEM components and instrumentation at Coherent Inc. in Santa Clara, Calif.; email: [email protected].