John Coates, Coates Consulting
One of the most important attributes of
infrared spectroscopy is its ability to handle physically small samples or small
features on samples. Important applications include forensic analysis of a crime
scene, where infinitesimal evidentiary samples are collected for identification
and/or characterization. Another consideration is the ability to isolate and characterize
cell defects, including the cancerous regions of a biological specimen.
In the early days of IR spectroscopy, the issue was how to handle
such small samples and how to get sufficient energy to them to enable recording
a useful spectrum. The options ranged from using relatively low cost microsampling
accessories, such as beam condensers, to specially configured microscope accessories.
For more than a decade, the role of the IR microscope has been
growing, and dedicated products are available that enable microscopic-scale samples
to be handled and characterized on a routine basis. Although these products can
be extremely expensive – ranging from approximately $40,000 to $200,000 –
they are one of the most valuable and cost-effective tools in the analytical arsenal.
More recently, the biggest advances have been in imaging areas, with either mapping
stages or imaging arrays, or combinations of both.
Until now, the application has been limited to standard Fourier
transform infrared (FTIR) instrumentation interfaced to standard microscope platforms
or adapted/customized microscopes optimized to handle atmospheric interference issues.
This article introduces a concept for IR microscopy based on a
broadly tunable IR quantum cascade laser (QCL), where the improved spectral radiance
of the laser provides more efficient optical coupling to smaller sampling areas,
which can translate to more efficient spectrally specific IR imaging. The LaserScope
from Block Engineering of Marlborough, Mass., is expected to enable the technology
to expand into new application areas, especially in bioscience research and medical
diagnostics.
IR microscopy: A history
There is nothing new or modern about the IR microscope; in 1953,
Vincent J. Coates (no relation) published a paper on the design and performance
of an IR microscope attachment.1 The attachment was the precursor to the first commercial
IR microscopy system, which was offered by Perkin-Elmer Inc. The microscope was
interfaced to a Model 12 single-beam dispersive spectrophotometer (Figure 1a).

Figure 1a. The original PerkinElmer Model 12-based infrared microscope was made circa 1953.
Courtesy of Coates Consulting.
In his publication, Coates cited applications including the measurement
of fibers, crystals and biological tissues, then a major milestone. However, relative
to modern spectroscopy and modern-day demeanor, this was not a practical solution
because of the very long time required to record a spectrum of good quality. It
was used as a specialist metric tool for certain high-tech industries but not employed
as a general-purpose analytical tool.
Not until 1983, with the introduction of Digilab LLC’s IRMA
(infrared microscope accessory), did the spectroscopist really become re-engaged
with IR microscopy. This system, designed to interface to an FTIR spectrometer,
provided a transmission-only method of measurement. Within about a year, it was
replaced by the company’s UMA-100, which included the option of reflectance
measurement. In the ensuing years, right up to the 2000s, we have seen iterations
of FTIR-based microscopy products, ranging from smaller in-compartment accessories,
such as the original Spectra-Tech Inc.’s Spectra-Scope (circa 1985), to a
fully integrated FTIR microscope, the IRμS, introduced by Spectra-Tech in the
1990s.
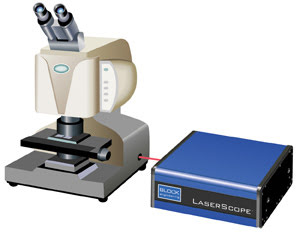
Figure 1b. The Block
Engineering LaserScope, a next-generation IR microscope, uses a broadly tunable
IR quantum cascade laser. Courtesy of Block Engineering.
Today, essentially all commercial FTIR manufacturers offer optimized
IR microscope systems, and IR microscopy has become a routine analytical tool. Most
of these systems provide some form of hyperspectral imaging, an extremely powerful
tool for advanced materials characterization, forensic identification and, most
importantly, medical diagnostics.
Researchers have long wanted to be able to study the chemistry
of biological tissue, and this has become a reality: Cell histology results, normally
subjectively provided based on the experience of the pathologist, can be validated
by the chemistry of the cell based on IR spectral imaging. The principle of this
measurement is summarized in Figure 2, where the false-color image illustrated in
Figure 2c represents the changing diagnostic chemistry of a diseased cancer cell.
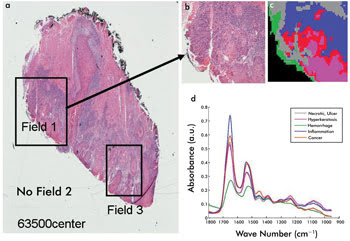
Figure 2. Cancer cell histology: Shown is a comparison of traditional
microscopy (a and b) and an IR microscopy-mapped image (c) and recorded spectra
(d). Courtesy of professor Max Diem, Northeastern University.
Working beyond the limits
The process of FTIR microscopic imaging involves serial movement
of the mapping stage of the microscope, where the IR beam is imaged to an area of
a few microns. On a conventional instrument, this process is slow because of the
high level of attenuation of the IR beam and the need to scan each spot location
to provide an optimum signal-to-noise ratio (SNR) from the conventional light source
of the spectrometer.
In recent work, high-performance imaging has been made possible
by the integration of the high brightness of the synchrotron light source with the
FTIR microscope. This has enabled high-resolution imaging in a practical time frame.
Although a good solution, it is practical only in a research environment, and not
everyone has a synchrotron in his/her backyard.
Although maybe not as high in total radiance, the QCL provides
higher spectral radiance than the synchrotron source and significantly more than
the standard FTIR source, as indicated in Figure 3. The spectral radiance, as a
function of wavelength of the QCL, is approximately seven orders of magnitude greater
than the FTIR source (note that the synchrotron is about three to four orders greater
in spectral radiance).
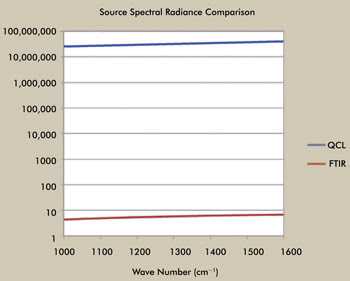
Figure 3. Comparison of the spectral radiance between the QCL laser and the FTIR light
source. Courtesy of Block Engineering.
The FTIR traditionally uses a conventional extended thermal source,
commonly in the form of an electrically heated ceramic element or rod. This limits
how small the focused image of the source can become, thereby limiting the area
that is efficiently imaged by the source without excessive throughput losses. On
the other hand, the QCL is a point source, and this can be imaged down to a small
spot without the associated losses of throughput.
The benefit of this source for handling small samples with reduced
focused beam size can be appreciated from Figure 4, which compares the relative
SNR, a practical measure of optical throughput, for FTIR versus the QCL. With a
sample size of 100 µm, the throughput of the two systems is comparable, in terms
of SNR. At 20 µm, the SNR is approximately 30 times greater for the QCL, which can
equate to better spectral quality for the reduced sample size and/or shorter acquisition
times.
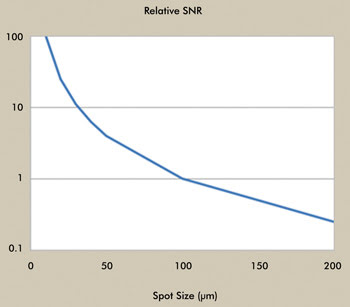
Figure 4. Comparison of relative SNR as a function of sample spot size, QCL vs. FTIR. Courtesy
of Block Engineering.
Laser-based spectroscopy
The QCL technology provides the opportunity to have the tunable
range from 6 (~1665 cm-1) to 12 µm (~830 cm-1). For many applications, this is the
sweet spot and covers most of the “fingerprint” region of the IR spectrum.
This is particularly the case for biological tissue, where one typically works in
an aqueous environment, and where the range indicated above falls conveniently within
an absorption window for water.
The QCL, combined with a microscope configured for infrared applications
(Figure 1b), offers significant benefits based on the greater brightness of this
source (Figure 3) and the improved SNR for an apertured sample image (Figure 4).
An example of the anticipated spectral quality for the imaged laser source is demonstrated
in Figure 5a for a sample of a polymer mounted behind a 20-µm pinhole (obtained
without microscope optics). This spectrum, gained in approximately 0.5 s, is of
high quality and compares qualitatively with the FTIR spectrum of a standard reference
sample of the polymer (Figure 5b).
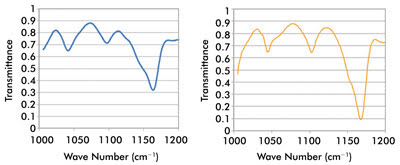
Figure 5. At left is the spectrum of a polymer film taken through a 20-μm pinhole;
at right, comparison of a standard FTIR reference spectrum of the polymer film material.
Courtesy of Block Engineering.
A major advantage is expected to be in chemical imaging. The source
brightness, although not the same as the synchrotron source, should provide much
of the performance gains experienced with the synchrotron source. Also, because
individual wavelengths can be scanned without having to record the entire spectrum
(the inverse multiplex advantage), the wavelength-specific information used for
chemical functionality mapping will enable higher-quality, higher-SNR spectra to
be obtained in much shorter time frames than the FTIR systems.
The QCL also does not require the liquid nitrogen cooling of the
standard MCT detector, as used on the FTIR microscope, which is not only an inconvenience
but imposes a constraint on the relatively long acquisition times experienced with
conventional sample mapping.
Conclusion
The integration of QCL with a microscope provides not only a next
generation of performance, especially for spectral imaging, but also an advantage
over the FTIR microscope with regard to packaging. An IR microscope takes up a lot
of benchtop real estate, and there is a relatively high operational overhead caused
by the need for liquid nitrogen cooling of the detector.
The system shown in Figure 1b has a lot less lab benchtop real
estate than the conventional FTIR microscope, and in the future, the laser’s
packaging will become smaller. It is expected that it will become physically integrated
into the microscope, much like a standard light source.
In a way, IR microscopy has come full circle from the dispersive
system first introduced in 1953 – but today, it is much more efficient and
more compact.
Meet the author
John Coates is principal consultant at Coates Consulting in Newtown,
Conn.; e-mail: [email protected].
Reference
1. V.J. Coates et al (1953). Design and performance of an infrared
microscope attachment. J Opt Soc Am, Vol. 43, pp. 984-989.