Laser-induced shock waves on neural cells, monitored by quantitative phase imaging, are helping to build understanding of brain damage and its physiological impacts.
NICOLAS PEREZ, PEGAH POULADIAN, AND DARYL PREECE, UNIVERSITY OF CALIFORNIA, IRVINE
Traumatic brain injury can have catastrophic impacts on everyday functioning and quality of life. Now, a set of photonic tools may provide fresh insights into this condition. Along with the technical knowledge made available by the application of the biological and physical sciences, new imaging methods and biophotonics techniques are being put to good use. A prime example of this interconnectivity is the study of laser-induced shock waves (LIS).
iStock.com/naratrip boonroung
The effects of shock waves on the human body were first noticed during World War II, when it was discovered that sailors who were in the ocean at the time of a torpedo explosion, but who were not targeted directly, often died from lung disruption. In a number of cases, they had suffered no external physical harm. More recently, though, shock waves have been implicated as a cause of traumatic brain injury. When shock waves created by an impact or blast wave travel through the skull, they can damage neural tissue. The damage to the brain may be lasting, leading to a variety of debilitating effects such as memory loss, depression, and other mental impairments.
To study the effects of shock waves on the brain, the authors’ research team at the University of California, Irvine recently used a pulsed 1064-nm Nd:YAG laser to create microscopic shock waves and monitor the effects on single cells. By using a pulsed laser beam focused on a single point, extremes of energy can be generated. When the buildup of energy at the focal point is strong enough, a cavitation bubble forms and then collapses, which generates a shock wave that radiates outward from the focus. By precisely controlling the beam parameters, shock waves can be calibrated to apply forces equivalent to those of known sources of traumatic brain injury.
The authors used this phenomenon to simulate brain injuries in vitro by using laser-induced shock waves to damage neuronal cells such as astrocytes. Astrocytes are star-shaped glial cells that are the most numerous cells in the central nervous system. They play a crucial role in maintaining the healthy functioning of neurons in the brain. Since the cells are responsible for maintaining and supplying nutrients to nervous tissue in the brain and spinal cord, studying how astrocytes respond to trauma lends valuable insights into the mechanisms that the brain uses to repair internal damage.
One way to assess the damage inflicted on a cell is to monitor morphological changes to the cell. By creating shock waves near a cell and quantitatively measuring the change in cell volume using holographic microscopy, it is possible to see how the cell changes shape in response to an applied external force.
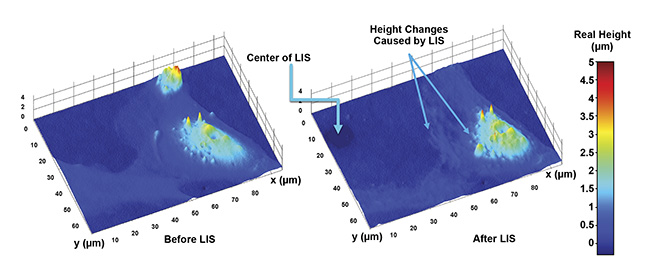
Laser-induced shock waves (LIS) in live astrocyte cells. The exact height changes caused by the event can be measured in various parts of the cells. Courtesy of University of California, Irvine.
Quantitative phase microscopy (QPM)1 is centered on phase interference imaging. Phase imaging dates back to 1887 with the invention of the Michelson interferometer. In the 1980s, these interference techniques were taken a step further with the addition of digital scanning technology that enabled dynamic holographic interferometry. The transparency of living cells has historically made them difficult to image under a bright-field microscope. However, QPM can image the surfaces of the cells with nanometer resolution by using the phase information of the light as it passes through the cells. QPM captures interference images resulting from two beams: one that passes through the sample while the other is used as the reference beam. By making use of phase-unwrapping algorithms, QPM can measure the phase shifts in light caused by the cell. By knowing the refractive index, the height of the cells can be quantitatively measured via the phase information.
For the purposes of the authors’ research, QPM was used to observe the thickness of neural cells. This was used to calculate the cells’ volume and any exchange of mass between the cells and their environment or neighboring cells. LIS was then used to induce cellular damage and simulate traumatic brain injury, so that the response of neurological astrocytes could be determined and the cells’ role in the process could be characterized.
By combining this work with conventional fluorescence techniques, a complex system of intercellular signaling has been gradually revealed. This signaling network, which changes intracellular calcium levels2, works to regulate the response to the damage to nearby astrocytes and mediates cellular apoptosis and repair.
Other techniques at work
Despite the insights provided by laser-induced shock waves and QPM, it is often advantageous to use less destructive techniques to manipulate cells under the microscope. Here, too, biophotonics technology has provided a way to move and probe neuronal cells.
Perhaps one of the most groundbreaking uses of laser beams at the cellular scale was Arthur Ashkin’s invention of optical tweezers, for which he won the Nobel Prize in 2018. Ashkin’s insight that radiation pressure could be used to confine tiny microscopic particles in three dimensions has enabled a plethora of measurements at the subcellular level, including in the brain. By using highly focused laser beams, Ashkin was able to create a so-called optical gradient force on dielectric particles close to the focus of the beam. This force enabled him to trap and move individual particles at a size scale never before achieved.
Both laser-induced shock waves and optical tweezers can be used to study mechanical effects on cells. Courtesy of University of California, Irvine.
Another important technique — the use of focused laser light to ablate cells — was pioneered in the early 1970s by Michael Berns, who showed that a focused laser beam could be used to dissect individual cells under a microscope. This technique, called cell surgery, demonstrated that light could be used not only for imaging but also as a tool for cutting cells. Together, these techniques enabled the movement and dissection of cells under the microscope. Thanks to these developments, the authors were able to use optical tweezers to manipulate particles around samples and to apply forces onto the cells and measure the effects. They were also able to selectively apply damage to specific parts of cells, such as to individual axons3.
Neuroscience moves forward
Modern neuroscience has been infused with optical technologies that are capable of providing high-resolution and high-speed information about neuronal processes deep at the cellular level, enabling the mapping of neuronal activity across the brain. Both in the brain and on the microscope slide, these new technologies have become increasingly relevant to the task of unraveling the mysteries of a brain’s structure and function.
The interplay between physics and biology has defined the field of biophotonics in neuroscience, now often called neurophotonics. Physical principles have contributed to the creation of new biological technology, while biological motivations and techniques have influenced new areas of interest and discoveries in the physical sciences. Neuroscience has thus benefited from the application of physical principles to problems in neurobiology.

Slices through an astrocyte cell show how the height of the cell changes spatially over its surface. From this, the volume of the cell can also be found. Courtesy of University of California, Irvine.
Some of the mystery surrounding traumatic brain injury, perhaps one of the most complex pathologies of the brain, has been dispelled thanks to biophotonic techniques that can mimic this type of injury at the cellular level. The effects of traumatic brain injury on astrocytes are still a relatively unknown piece in the functioning and development of the brain, but with the tools that researchers now have at their disposal, they may be able to uncover many mysteries that could shed light on how the medical community could prevent or reverse neurological injuries.
Meet the authors
Nicolas Perez is a doctoral candidate in biomedical engineering at the University of California, Irvine. His research interests include the application of optical technology to solve problems in biology. His work focuses on optical vortices, spectroscopy, and bio solitons; email: [email protected].
Pegah Pouladian is a doctoral candidate in biomedical engineering at the University of California, Irvine. Her research interests include the damage and repair mechanisms of neuronal cells. Her work focuses on quantitative phase microscopy for the study of traumatic brain injury; email: [email protected].
Daryl Preece is assistant professor at the University of California, Irvine’s Department of Biomedical Engineering and a member of the Beckman Laser Institute. His research — which spans fundamental optics to translational biophotonics — centers on how light interacts with matter and the optically generated forces that occur when it does. He has particular interest in the use of biophotonics technologies at the cellular level; email: [email protected].
References
1. P. Pouladian et al. (2021). Combining quantitative phase microscopy and laser-induced shockwave for the study of cell injury. Biomed Opt Express, Vol. 12, No. 7, p. 4020, www.doi.org/10.1364/boe.427693.
2. V. Gomez-Godinez et al. (2015). Laser-induced shockwave paired with FRET: a method to study cell signaling. Microsc Res Tech, Vol. 78, pp. 195-199, www.doi.org/10.1002/jemt.22463.
3. A. Selfridge et al. (2015). Rat embryonic hippocampus and induced pluripotent stem cell derived cultured neurons recover from laser-induced subaxotomy. Neurophotonics, Vol. 2, p. 015006, www.doi.org/10.1117/1.nph.2.1.015006.