From in situ investigations to in vivo imaging, researchers have embraced nanotechnology to help them understand the intricacies of the human body at the smallest scale.
Lauren I. Rugani, Contributing Editor
Nanotechnology is becoming increasingly popular in the medical field, both in imaging and in direct treatment applications. The risks associated with nanotechnology are prompting researchers and scientists to develop safe ways to investigate cellular functions and to treat abnormalities so even the smallest biological systems can be better understood.
Stem cell stimulation
Peidong Yang and a group from the University of California, Berkeley, and from Gladstone Institute of Cardiovascular Disease in San Francisco are exploring the practicality of interfacing nanowires with mammalian cells as a first step in electrically stimulated stem cell differentiation.
Nanowires with a high aspect ratio are ideal for probing the biological processes that occur within cells, across cell membranes and between neighboring cells. The silicon nanowires used by the researchers were ~90 nm in diameter and up to 6 mm long — much smaller than the 10-mm-diameter mammalian cells of interest. Such access to the interior of the cells may enable the study of complex regulatory and signaling patterns.
As detailed in the June 13 issue of the Journal of the American Chemical Society, arrays of nanowires were synthesized vertically on silicon substrates through a chemical vapor deposition process. Each array had nanowires of a different diameter. The researchers then cultured mouse embryonic cells expressing GFP on the high-density nanowire arrays.
Physical interaction between the nanowires and the cells was visualized by a variety of techniques. Confocal microscopy images revealed the nanowires as small black dots within the GFP-expressing cells. Scanning electron microscopy images captured the actual penetration of individual cells by the nanowires (Figure 1). Because of the small diameters and high aspect ratios of the nanowires, no external force was necessary to promote penetration — which occurred naturally within 1 h — as observed by fluorescence microscopy.
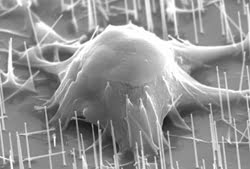
Figure 1. Scanning electron microscopy images demonstrate the penetration of individual mouse embryonic cells by 90-nm-diameter nanowires. No external force is necessary to facilitate penetration because of the small diameter and high aspect ratio of the nanowires.
The investigators then studied the proliferation of the cells on the nanowire array. After 1 h in the cell medium, they moved the substrates to a fresh medium that did not contain cells, ensuring that the test would be performed only on the cells penetrated by nanowires. The density of the array was reduced so that each cell was penetrated by an average of two to three nanowires.
The researchers found that cell viability depended upon the diameter of the nanowires that penetrated them. “This might be a combination of physical volume and chemical interaction,” Yang noted. Cell death occurred within the same day of penetration by nanowires 400 nm in diameter, while cells survived for up to five days when penetrated by nanowires with diameters of only 30 nm. Fluorescence microscopy images showed that 78 percent of cells penetrated by nanowires with a 90-nm diameter remained healthy for up to two days.
They noted that a small diameter is a critical element for studying intracellular processes. Yang suggests that future studies could investigate cell dependence on other factors, including nanowire length, depth of penetration and the number of penetrating nanowires, as well as the effect of the penetration on cellular function.
The scientists also found that the array containing 90-nm-diameter silicon nanowires was more efficient at cell retention than both conventional gelatin-coated plates and plain silicon substrates containing no nanowires.
After the substrates incubated in a cell medium for 1 h, the group placed them into a fresh medium and found that the nanowire array held twice as many embryonic cells as both the gelatin and flat silicon substrates. This discovery marks the nanowire array as a useful apparatus for maintaining the differentiated cells.
After seven days of differentiation, GFP-expressing cells were placed onto the silicon nanowire substrates. The number of cells expressing GFP increased through day 10, indicating proliferation and further differentiation on the substrates.
Finally, the researchers tested the silicon nanowire array’s suitability as a tool for gene delivery. Human embryonic kidney cells were cultured on substrates with electrostatically deposited DNA containing GFP. After one day, less than 1 percent of the cells expressed GFP, indicating the successful delivery and normal functioning of the gene.
Yang and his group plan to investigate chemical delivery and DNA extraction with high spatial resolution as well as to study the effect of electrical stimulation on cell differentiation. “We’d like to explore this new version of the nanoelectrode and see if we can guide how stem cells differentiate,” he said. “It would represent a new way of manipulating stem cells to form tissues.”
Flipping the switch
Understanding and manipulating the behavior of neurons is a significant area of study in neurology. Researchers at MIT in Cambridge, Mass., have devised a method for activating and inactivating neurons with light to better understand their precise roles in neurological functions.
“By turning off a neuron for a precise amount of time, it becomes possible to know not only what the function of that neuron is for a particular behavior but when and how it makes its contribution,” said Edward S. Boyden, leader of the research group at the MIT Media Lab.
In a previous study, Boyden and his colleagues achieved photostimulation of neurons with blue light; they reported in the March 2007 issue of PLOS One that they could also induce photoinhibition, this time with yellow light. The ability to activate and silence the same neuron will enable scientists to determine the sufficiency and necessity of a single neuron for a specific behavior.
A class of molecules called halorhodopsins is known for responding to yellow light by pumping negative ions into a cell, thus reducing the membrane potential. The researchers fabricated a fusion protein containing an optimized form of halorhodopsin as well as a fluorescent protein that enabled it to be seen easily when expressed in cultured hippocampal neurons. The neurons experienced outward currents of 88.7 ±32.8 pA when exposed to 10 mW/mm2 yellow light from a xenon lamp, with onset and offset times of ~10 ms. Neither of these values varied considerably when tested at holding voltages of –70, –30 and 10 mV. In response to 1-s pulses of yellow light separated by a 1-s pause, the neurons reached peak hyperpolarizations of 32.9 ±14.4 mV.
Through a variety of control experiments, the group saw no difference in potential or membrane resistance between neurons expressing the halorhodopsin protein and neighboring neurons, indicating that neural activity in the presence of halorhodopsin would be relatively unaffected in the absence of light. Furthermore, they found good agreement in timing and amplitude for repeated trials on one neuron, across several neurons and over a period of time, suggesting that the mechanism could accurately simulate synaptic activity.
To show that halorhodopsin-expressing neurons could be silenced with light, the group introduced trains of 4-ms, 300-pA somatic currents to neurons, which fired 20 action potentials at 5 Hz. They then delivered yellow light pulses to silence specific spikes, allowing activity to resume immediately after the light was removed. The rapid onset and offset times of halorhodopsin allowed even single spikes to be blocked. However, exposure to continuous yellow light caused the hyperpolarization peak amplitudes to decrease to about 50 percent of the original values — evidence that the halorhodopsin had entered into a long-lasting inactive state.
Boyden recalled an earlier paper on halorhodopsin kinetics and hypothesized that pulses of blue light could accelerate recovery of halorhodopsin, rapidly enabling hyperpolarizations comparable to those achieved during the initial trials. Although blue light pulses aided the restoration of halorhodopsin, they also could activate neurons expressing another molecule called channelrhodopsin-2, which works similarly by allowing positive ions into the cell. By simultaneously expressing the two molecules in a single neuron, alternating pulses of blue and yellow light could facilitate bidirectional control of voltage (Figure 2).
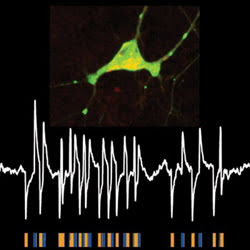
Figure 2. A neuron expressing both light-activated molecules — channelrhodopsin-2 and halorhodopsin (top) — allows its voltage to be controlled bidirectionally with two colors of light. Alternating yellow and blue light pulses correspond to hyperpolarization and depolarization events (bottom).
“Reprogramming neurons with two different colors of light allows very fine control of neural activity, allowing, for example, the ability to precisely alter the timing of neuronal spikes,” Boyden suggested. The group generated trains of precisely timed spikes by injecting randomly varying currents into neurons expressing both halorhodopsin and channelrhodopsin-2. In trials with concurrent illumination with alternating blue and yellow light pulses, the two molecules could delete spikes, add spikes or alter the timing of the spikes. Such manipulations could occur without altering the overall spike rate — a tool not yet accomplished by existing technologies but crucial for understanding the role of synchronous neural activity in the brain.
The group is working to develop new hardware, including optical fiber arrays, which will allow the technique to be applied to structures deep within the brain (Figure 3).
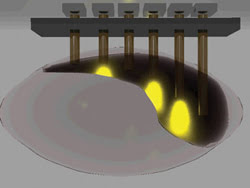
Figure 3. A three-dimensional rendering of an optical fiber array demonstrates light scattering through the brain as the cells are silenced with pulses of yellow light. An array featuring blue light would stimulate the cells.
Vascular visualization
Nanoparticles also have emerged as tools for imaging features within the brain. Dr. Edward A. Neuwelt and his team from Oregon Health & Science University in Portland, along with colleagues from the University of Utah in Salt Lake City and the National Cancer Institute in Bethesda, Md., investigated the ability of iron-oxide nanoparticles to image malignant brain tumors, as reported in the April 2007 issue of Neurosurgery.
The group compared ferumoxytol, a form of iron-oxide nanoparticles, with the traditional contrast agent gadolinium in dynamic and postcontrast MRI on 12 patients with brain malignancies. The patients underwent a series of imaging procedures — beginning with an MRI scan in the absence of a contrast agent — once with the administration of gadolinium and again with ferumoxytol.
For brain perfusion imaging, rapid MRI sequences were applied during contrast bolus administration. Using gadolinium, the signal intensity reached its equilibrium phase gradually in the regions of leaky tumor tissue, suggesting extravasation of the contrast agent. This phenomenon was not found with ferumoxytol. “Ferumoxytol remains intravascular and does not leak across even the damaged blood-brain barrier, allowing better vascular characterization,” Neuwelt said.
The researchers monitored the contrast uptake in regions in and around the tumor as well as in control areas of the brain by evaluating changes in T1 values, which are inversely proportional to the tissue-contrast agent concentration. Minutes after contrast administration, only gadolinium showed a significant increase in tissue concentration, confirming that ferumoxytol leakage is not significant in the early phase.
Time-of-flight magnetic resonance angiography was performed within 20 min after injection. Ferumoxytol highlighted more vascular structures within the brain than gadolinium did. The larger size of the nanoparticles allows them to remain inside vascular structures longer than gadolinium, which permeates the blood-brain barrier much more quickly. This leakage causes gadolinium-enhanced images to illuminate the tumor better than ferumoxytol but obscures vascular imaging (Figure 4).
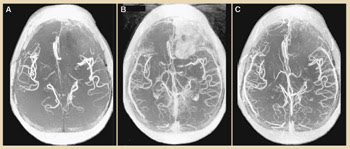
Figure 4. Time-of-flight angiography shows blood vessels in the brain without contrast (A), 15 min after gadolinium administration (B) and 15 min after ferumoxytol administration (C). Gadolinium enhances the tumor image, but more vessels are visible with ferumoxytol. Image reprinted with permission of Neurosurgery.
The investigators employed multiple MRI scans on the patients at 4 to 6 h, 16 to 20 h, 24 to 28 h, 48 to 52 h, and longer than 72 h after contrast administration to analyze the time-delayed enhancement of ferumoxytol. They acquired 3-mm-thick axial slices using both GE Medical Systems 1.5-T and Philips Intera 3-T MRI scanners.
The peak enhancement was found between 24 and 28 h after contrast administration in both magnetic field strengths. The average signal intensity decreased after 28 h but was still evident after 72 h, compared with gadolinium, which displayed maximum enhancement only 25 min after injection.
Because ferumoxytol leaves vascular structures much more slowly than gadolinium, it is ideal for dynamic imaging techniques such as perfusion because the assessment of blood volume and mean transit time assumes no contrast agent leakage out of blood vessels. Although gadolinium may help to reveal the size of a tumor, ferumoxytol visualizes the vascular structures within the tumor, which might respond to treatment before the tumor begins to shrink. Neuwelt believes that the nanoparticle contrast agents will help to detect such early therapeutic changes in brain tumors using dynamic MR imaging.
Nanoparticles also are useful as a contrast agent during surgery. The delayed enhancement could allow visualization of residual tumor tissue after an operation, without the difficulties of repeated contrast administration as experienced with gadolinium. Ferumoxytol can help to enhance inflammatory lesions as well as tumor cells distributed beyond the area imaged by gadolinium.
Clinical trials have shown that the possibility of an adverse reaction to the contrast agent is very low. Because of its low toxicity, ferumoxytol is being tested as an iron supplement therapy agent and could prove beneficial during intraoperative MRI because of its long plasma half-life.