A compact and portable imaging platform, aided by a femtosecond fiber laser, tracks disease progression and therapeutic results in a clinical setting.
MIHAELA BALU UNIVERSITY OF CALIFORNIA, IRVINE
Skin imaging at the cellular level is critical for understanding how diseases and aging affect the skin, and for developing strategies and therapies to prevent and combat these effects in a clinical setting. Visualizing dynamic cellular and molecular processes during the progression of skin disease and throughout the course of therapy requires an effective compact and portable imaging tool that can provide noninvasive imaging of wide (millimeter to centimeter scale) skin areas, rapid scanning and image acquisition time, submicron resolution, and label-free molecular contrast from multiple sources to enhance imaging specificity.
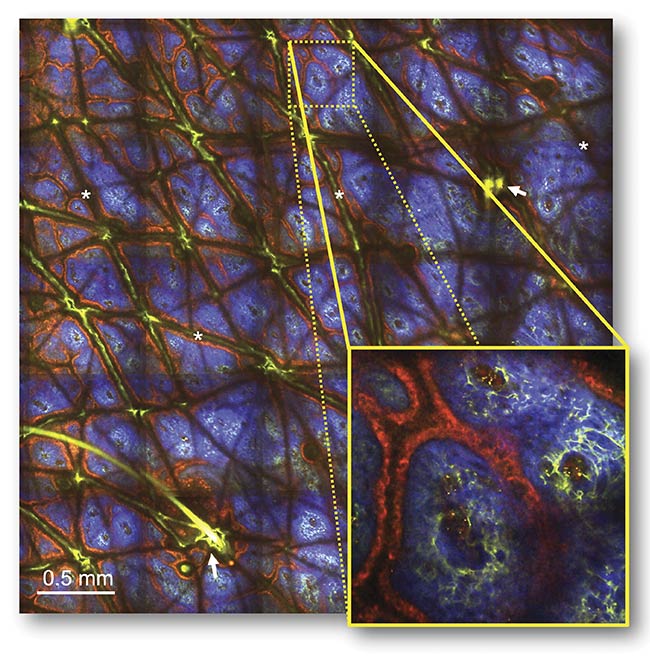
A macroscopic image with microscopic resolution, taken from the forearm of a volunteer and generated by a fast-scanning, large-area multiphoton exoscope (FLAME). The time-resolved 24-MP image (measuring 3.8 × 3.8 sq mm) — acquired at 2.4-μs pixel dwell time and at 60-μm depth in the dermoepidermal junction — shows pigmented keratinocytes (red) surrounding the collagen (blue) and elastin (green) fibers within dermal papilla. Skin folds (*) and hair follicles (arrows). Courtesy of UC Irvine.
Multiphoton microscopy is a laser-scanning imaging technology that has the potential to meet these needs, owing to its ability to generate depth-resolved images of living tissues in their native environment with histologic resolution and sensitivity based on endogenous molecular and chemical contrast. In multiphoton microscopy of skin, the contrast is based on second-harmonic generation imaging of collagen and two-photon-excited fluorescence of autofluorescent cofactors, such as reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD), elastin, keratin, and melanin. In addition to the specificity provided by detecting the second-harmonic generation signal from collagen, multiphoton microscopy can visualize specific skin fluorophores based on their fluorescence lifetime detection.
Scanning wide areas along the skin surface allows visualization of the dynamics of a single-cell and entire cell populations across a lesion or over a region of interest from the context of skin heterogeneity. Enhancing scanning speed improves the efficiency of the imaging procedure, minimizes motion artifacts, and optimizes the clinical workflow. Submicron resolution is required to identify cellular and fibrillar structures, along with submicron-scale cellular features such as melanocytic dendrites, which contain microtubule structures that transport pigment. In vivo label-free imaging captures physiological cellular behavior in tissue but provides limited molecular specificity. This limitation requires strategies for enhancing the selective detection of specific fluorophores to facilitate image interpretation.
The potential of using multiphoton microscopy for human skin imaging has been demonstrated in several clinical research studies that emphasized the need for key technological advancements to enable the broad adoption of this unique imaging approach1-3. Recent breakthroughs made at the University of California, Irvine (UC Irvine) demonstrate (in pilot studies) a compact, fast-scanning, large-area multiphoton exoscope (FLAME) that enhances the readiness of the technology for dissemination and testing in clinical trials4,5.
Compactness and portability
The size and power of the laser source are critical to designing a truly compact and portable microscope. Femtosecond fiber lasers have the potential to provide small, cost-effective alternatives to the bulky and expensive Ti:sapphire-based systems used in most of the current multiphoton microscopy applications. However, achieving the reproducible and stable laser performance that is required for in vivo multiphoton microscopy in a small fiber-based architecture necessitates a novel optical design and innovative thermomechanical packaging.
In conjunction with ongoing research in multiphoton microscopy at UC Irvine, Calmar Laser Inc. developed a compact femtosecond fiber laser that provides sufficient power (>500 mW at 780 nm) to compensate for the optical inefficiencies of the microscope setup. The lack of wavelength tunability in this design is more than compensated for by its many benefits. The compact laser head (560 cm3) is more than 70× smaller than a Ti:sapphire laser. Its size, along with an armored fiber cable connection to a rack-mounted power supply, enables the laser’s incorporation directly into the imaging head. This approach eliminates the need for an optical articulated arm to steer the beam into the imaging head, as well as the need for a beam control feedback mechanism to realign the beam after relocation of the imaging head.
The imaging head can house, along with the fiber laser, the required optics and optomechanics for laser scanning, beam expansion, and power attenuation, and a closed-loop servo-controlled miniature translation stage to allow integration of a translatable field of view (FOV) with high precision. Further, an all air-cooled fiber laser design obviates the need for a chiller. These benefits equate to lower cost and reduced overall complexity, while allowing enhanced compactness and portability of the imaging platform (Figure 1).

Figure 1. FLAME, a compact and portable multiphoton microscope-based imaging platform, incorporating the Carmel X-780 laser and highly optimized for noninvasive clinical skin imaging. The platform was designed and developed at the Beckman Laser Institute & Medical Clinic at the University of California, Irvine. Photos by Paul Kennedy. Courtesy of UC Irvine.
Imaging wide skin areas
A common limitation of the FOV in conventional laser-scanning microscopes, in which the scanning mirrors are placed in proximity, is produced by the lateral motion of the laser beam at the back aperture of the objective. This configuration results in the displacement of the beam by the first mirror onto the second mirror, which, for large angles, can lead to vignetting and reduction of the FOV.
One solution to this dilemma is to employ a relay lens system between the scanning mirrors. In addition, custom-
designed lenses help to minimize the optical aberrations caused by the large scanning angles. But, besides the optimized single FOV imaging capability, the microscope must have the ability to image over skin areas on the millimeter to centimeter scale. This capability is enabled by scanning methods referred to as “tile and strip mosaic”6, where the patient’s skin is mechanically translated by a linear stage and an attached metallic ring, such that adjacent FOVs are stitched together to generate depth-resolved images over large areas.
Enhancing the scanning speed
Rapid scanning results in fewer detected photons to generate the image, which is a particular challenge for label-free multiphoton microscopy imaging of skin that generates images by detecting weak endogenous signals. One solution to compensate for the limited number of detected photons is to use a computational approach such as content-aware image restoration6. In this method, pairs of images can be acquired at low and high signal-to-noise ratios (SNRs) to train a sample-specific convolutional neural network by using the high SNR images as ground truth. The trained network is then applied to restore the low SNR images to the predicted high SNR images.
Keeping subcellular resolution
While rapid imaging of large areas of human skin is critical for enhancing the efficacy and clinical utility of multiphoton microscopy technology, it is essential to accomplish this goal while maintaining subcellular resolution. Olympus’ recent development of lenses with low magnification, high numerical aperture (NA), and long working distance (WD) objective — such as the company’s 25×, 1.05-NA, 2-mm-WD water-immersion lenses — has facilitated this goal. Provided that the lenses for relaying and expanding the laser beam at the back aperture of the objective are custom-designed to minimize the large-angle optical aberrations, this objective can provide the submicron resolution needed to ensure that cellular and fibrillar structures can be resolved.
Enhancing molecular contrast
Capturing depth-resolved subsurface images of skin with subcellular resolution over large areas significantly increases the sensitivity of feature detection. As a label-free imaging approach, however, multiphoton microscopy has inherent limited specificity. The intensity-based method commonly provides selective detection of the collagen through the second-harmonic generation signal. The two-photon-excited fluorescence signals of the elastin fibers — and of the NADH, FAD, keratin, and melanin used for the visualization of the skin cellular morphology — have significant spectral overlap and cannot be easily separated from the resulting image, complicating image interpretation.
A solution for selective imaging of specific skin fluorophores can be found in their fluorescence lifetime detection. Time-correlated single-photon counting detection is a conventional approach that enables acquisition of fluorescence lifetime decays with high temporal resolution, but the required long integration times in the clinical workflow make this approach impractical. An alternative solution is to digitize the analog output signal of the detector at a faster rate and bin the emitted photons into virtual detection channels based on their arrival time.
This approach results in reduced temporal resolution (hundreds of picoseconds) compared to conventional methods, but it is sufficient for the selective detection of some key skin fluorophores. Thus, melanin can be detected with high specificity based on its short fluorescence lifetime with respect to the other skin fluorophores (see image on page 26). Selective detection of melanin facilitates pigment quantification and allows imaging of melanocytes, melanoma cells, and melanin-rich macrophage infiltrates with high specificity (Figure 2). NAD(P)H (nicotinamide adenine dinucleotide phosphate) can potentially be detected using a similar approach, facilitating measurements of cellular metabolic changes associated with malignancy.
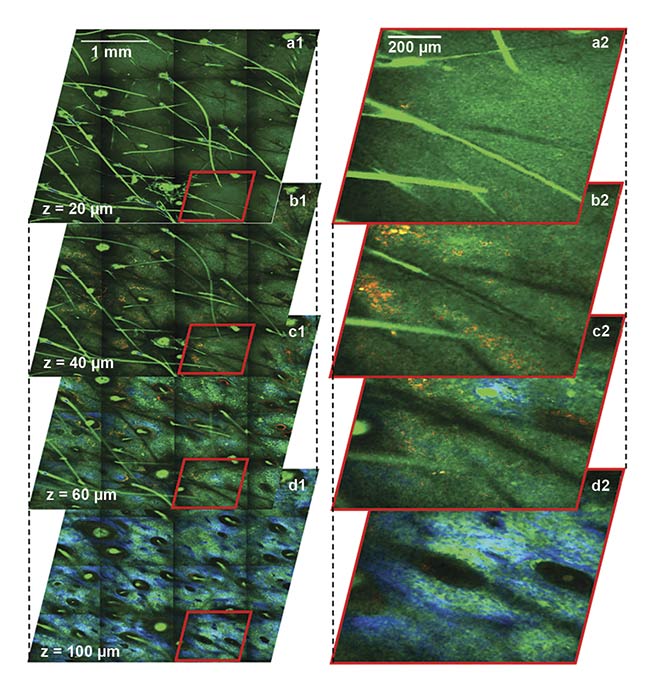
Figure 2. Depth-resolved images of
human facial skin captured using the
clinical FLAME device. The time-resolved macroscopic (a1-d1) and close-up images (a2-d2) show epidermal keratinocytes (a1-c1, a2-c2) as nonpigmented (TPEF, green) and pigmented (TPEF, red) cells,
collagen (SHG, blue), and elastin (TPEF, green) fibers (d1, d2). Hairs (TPEF, bright green) are visualized longitudinally and cross-sectionally at all depths. The volumetric images (a1-d1) were acquired in ~3 min. Images are color-coded by short-lifetime (<1.6 ps) TPEF (red) and long-lifetime (>1.6 ps) TPEF (green). TPEF: two-photon-excited fluorescence; SHG: second-harmonic generation. Courtesy of UC Irvine.
Figure 3 shows an example of images illustrating melanoma cells (malignant melanocytes) selectively detected in a melanoma lesion of human skin by the clinical multiphoton microscopy-based FLAME device. The images also highlight the nonuniform distribution of the melanoma cells across the lesion, emphasizing the critical need for a large sampling size to capture the lesion heterogeneity and avoid a false-negative diagnosis.
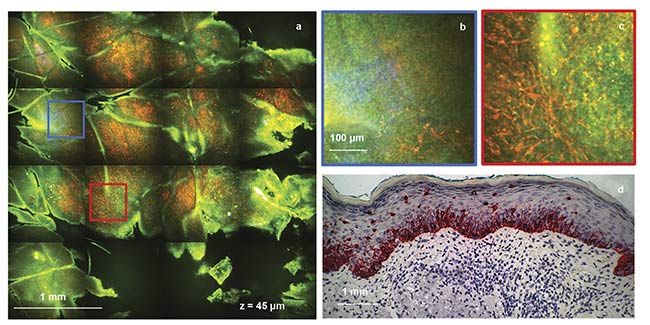
Figure 3. Images of melanoma in human skin, captured by the FLAME device. A time-resolved macroscopic image (measuring 3.5 × 3.5 sq mm) acquired at
a depth of 45 μm in ~50 s (a). Digital zoom of the blue outlined area in (a), showing a limited number of migrating melanocytes (red) (b). Digital zoom of
the red outlined area in (a), showing a large population of migrating melanocytes (red) (c). Images are color-coded by short-lifetime (<1.6 ps) TPEF (red) and long-lifetime (>1.6 ps) TPEF (green). For comparison, a histological section of the same lesion showing Melan-A positive melanocytes (red) and their migration
in the upper epidermal layers (d). Courtesy of UC Irvine.
Further developments
A multiphoton microscopy clinical platform, uniquely equipped with the necessary combination of features — such as a compact laser, expanded FOV and resolution, and both sensitivity and label-free specificity — has the potential to broaden the reach of clinical applications of skin imaging and image-guided therapy in two ways. First, such a platform could open up new methodologies for real-time detection, diagnosis, and therapy monitoring of skin diseases — methodologies that are pain-free and patient-friendly and that reduce time to treatment. Second, it could set the stage for more advanced translational studies that take full advantage of multiphoton microscopy’s capabilities.
With technical barriers reduced, examination of human subjects could become more routine, allowing the enrollment of greater numbers of patients and accelerating the standardization and validation of multiphoton microscopy. The FLAME imaging platform developed at UC Irvine to incorporate the aforementioned critical features is currently being used in clinical pilot studies to evaluate its potential for noninvasive early diagnosis of skin cancers and for monitoring the effects of various therapies on skin morphology and functionality.
Meet the author
Mihaela Balu, Ph.D., is an associate researcher and principal investigator at the Beckman Laser Institute & Medical Clinic at the University of California, Irvine. She has an educational background in physics and optical engineering from the University of Bucharest in Romania and from CREOL, The College of Optics and Photonics at the University of Central Florida. Her laboratory is focusing on the development and clinical translation of modern biophotonics technologies, such as nonlinear optical microscopy; email: [email protected].
Acknowledgments
This work was supported by the National Institute of Biomedical Imaging and Bioengineering (R01EB026705) and the UCI Skin Biology Resource-Based Center (P30AR075047). The author thanks the former and current members of her research group, particularly Alexander Fast, Ph.D., and Juvinch Vicente, Ph.D., for their valuable contributions to the work presented here.
References
1. M. Balu (2014). Distinguishing between benign and malignant melanocytic nevi by in vivo multiphoton microscopy. Cancer Res, Vol. 74, No. 10, pp. 2688-97, www.doi.org/10.1158/0008-5472.can-13-2582.
2. G. Lentsch (2019). In vivo multiphoton microscopy of melasma. Pigm Cell Melanoma Res, Vol. 32, No. 3, pp. 403-411, www.doi.org/10.1111/pcmr.12756.
3. R.B. Saager (2015). In vivo measurements of cutaneous melanin across spatial scales: using multiphoton microscopy and spatial frequency domain spectroscopy. J Biomed Opt, Vol. 20, No. 6, p. 066005, www.doi.org/10.1117/1.jbo.20.6.066005.
4. A. Fast (2020). Fast, large area multiphoton exoscope (FLAME) for macroscopic imaging with microscopic resolution of human skin. Sci Rep, Vol. 10, No. 1, p. 18093, www.doi.org/10.1038/s41598-020-75172-9.
5. M. Balu et al. (2020). University of California, Irvine, assignee. Imaging platform based on nonlinear optical microscopy for rapid scanning large areas of tissue. U.S. Patent Application Number 10,595,770 B22020. U.S. Patent and Trademark Office.
6. S. Abeytunge et al. (2011). Rapid confocal imaging of large areas of excised tissue with strip mosaicing. J Biomed Opt, Vol. 16, No. 5, p. 050504, www.doi.org/10.1117/1.3582335.
7. M. Weigert et al. (2018). Content-aware image restoration: pushing the limits of fluorescence microscopy. Nat Methods, Vol. 15, No. 12, pp. 1090-1097, www.doi.org/10.1038/s41592-018-0216-7.