Specially configured microscopes, along with illumination and analytical software, trace cell division and migration, revealing functional transformation and the success of drug therapies.
Scientists are increasingly turning to live-cell imaging to track changes at the developmental stage of life, which is vital for the advancement of cellular biology, and to grow and monitor proliferating stem cells and cancer cells in a contained environment. This research can provide clues as to how organisms evolve structurally and how therapeutics take hold before a disease requires invasive and life-altering treatment.
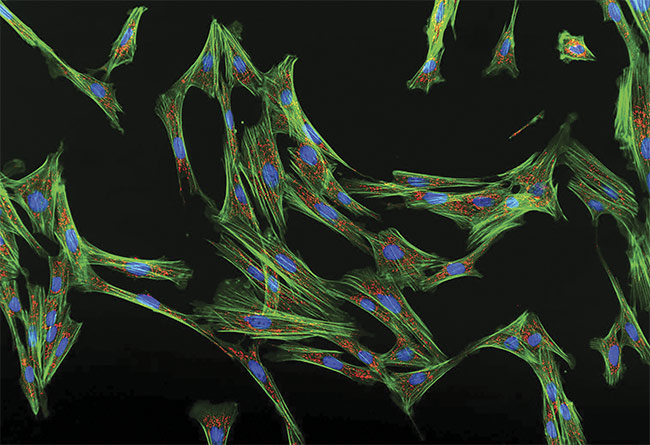
An image of fluorescent cell fibroblasts taken with the APX100 digital imaging system. Courtesy of Evident Scientific.
Live-cell imaging includes modalities such as widefield fluorescence, total internal reflection, Förster resonance energy transfer, superresolution, and time-lapse microscopies. The goal of the technique is to achieve direct insight into cellular behavior, often in 3D. But to garner those insights, a system designed with optimal automation, light sources, and stability must be constructed with AI to categorize the assembled data.
The global market for live-cell imaging systems is expected to reach $4.3 billion by 2028, rising from $2.7 billion this year, at a compound annual growth rate of 10.2%, according to a report from Markets and Markets. This growth is driven by improved microscopes as well as advancements in detector technology that is making live-cell imaging viable in drug discovery and cell biology. The largest technology segment for live-cell imaging is in time-lapse techniques.
The challenge for researchers is not only to capture and evaluate the functions of a living cell, but also to see how it changes over time (with measurements coming at set intervals and analyzed via AI software), especially when influenced by circumstances including disease or drug treatment. So, it is critical to house a sample culture in a stable environment.
One device that is designed to allow extended time for live-cell research is the CM30 incubation monitoring system, produced by Evident Scientific, which maintains the health of the cells researchers are studying because it does not require the transfer of samples to and from a storage area for examination. It is compatible with many sample repositories, such as petri dishes, microplates, and flasks.
The system illuminates a sample with LED light at 630 nm, which reduces the level of phototoxicity common in other microscopes, such as standard brightfield microscopes, and also allows the collection of data without labeling or staining the cells. And accompanying software provides consistent measurement of cell count, growth rate, confluency (density), and other statistics that can be monitored remotely during an experiment.
“Any lab with cell cultures wants to monitor for optimal confluency, because these are often very precious cell lines they’re working with, such as stem cells or cancer cells,” said Avi Smith, an associate product manager in the life sciences division of Evident Scientific. “And researchers require an approach as reproducible as possible. There are other cell incubation monitors on the market, but the key is in the epi-oblique illumination technique, which allows for the device to easily integrate into existing culture workflows.”
In oblique illumination, light is projected sideways and data is captured from a tilted plane without requiring moving the sample. In epi-oblique illumination, illumination and reflected light are delivered through the same objective (Figure 1). Smith said the direction of light is maximized, as opposed to blasting the sample with as many photons as possible.
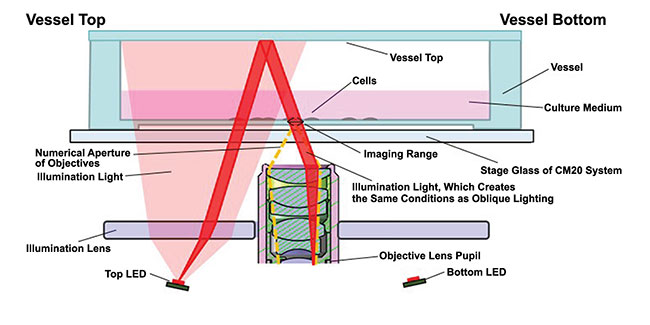
Figure 1. An illustration of the epi-oblique illumination method. Courtesy of Evident Scientific.
“Rather than hitting an incubator with external light, the illumination is moved inside, in the case of the epi-oblique illumination used in our incubator,” Smith said. “The system is compatible with many brightfield time-lapse methods.”
Jonathan Garlick, a professor in the Divison of Cancer Biology and Tissue Engineering at Tufts University, recognizes the importance of this capability when the goal in a lab environment is to monitor culture conditions with an eye toward reproducible results.
“There can be incredible heterogeneity in cells, and ideal confluency must be met because if it grows too much, often the sample will take on other functions or attributes,” he said. “I do a lot of skin research, and patients let us keep their cell samples. We might be isolating fibroblasts, for example, and we need to make sure the proper cells are grown out so we can correctly analyze the treatment.”
And conducive to a variety of extended analyses, Evident created the APX100 digital imaging system, with two configurations designed for varying levels of acquisition and analysis.
The instrument contains built-in Kohler illumination (even lighting), motorized aberration correction, and automatic sample detection via pretrained AI. The system is additionally capable of various types of contrast imaging and can perform live-cell time-lapse imaging and deconvolution. The APX100 has been used to examine multiplying cancer cells, bone marrow samples, and fibroblasts (opening image).
2D into 3D
Oblique illumination is also a principal driver behind other systems, such as Edge, a 3D microscope created by Edge-3D. Gary Greenberg, CEO of Edge-3D, said the technique is particularly effective for live-cell imaging because it captures contrast and highlights the components of a sample. This form of improved visualization can be a clear indication of a particular stage of development, disease, or treatment within a cell. He pointed out that the often-used differential interference contrast optical system produces similar highlights and shadows in the specimen but that this method is not suitable for use with plastic tissue culture dishes. This makes oblique illumination a better system for live-cell imaging.
Systems using oblique illumination can image samples such as 3D tissue culture and organ culture (Figure 2). Adaptable components can convert standard 2D microscopes into instruments for 3D visualization, which can reveal important clues in the fields of neuroscience, vascular research, and pathology, among others.
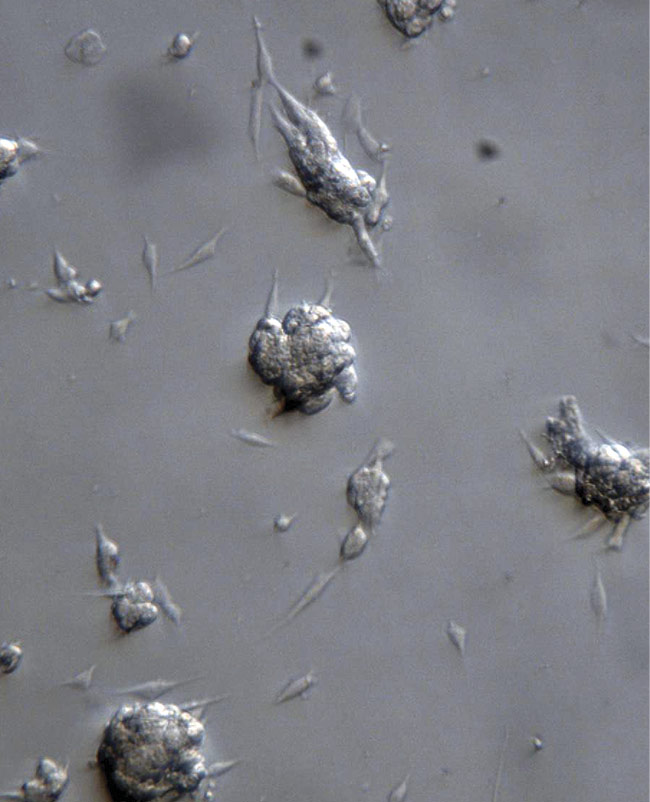
Figure 2. Cancer cells in culture, imaged with the Edge oblique illuminator. Courtesy of Edge-3D.
One key advantage of this technology is that while high-magnification microscopes typically have a smaller depth of field, and thus do not capture information from entire cells, the reconstruction software linked to the Edge line enables automated z-stacking and can be used to examine fewer, thicker sections of samples, or un-sectioned samples, such as live cells.
“With this method, stacking takes only a few seconds,” Greenberg said. “The reconstituted stacks can be put together to produce a time-lapse video with both spatial and temporal specificity.”
He said that the technology, which is prototyped by Edge-3D and mass produced by Prior Scientific, has potential applications in additional specialties such as oceanography and plant biology.
“Viewing specimens in 3D is useful for educational purposes, in general, because people see and understand things in 3D,” Greenberg said. “When you’re looking for contrast and interrelationships, a 2D image makes no sense.”
Multiple data points
Integral to a true picture of a live-cell sample is the ability to capture multiple points of reference with spatial and temporal clarity, while causing minimal photodamage. And during the past few years, the European Molecular Biology Laboratory (EMBL) developed a technique called line-scanning Brillouin microscopy to accomplish this, in the context of ongoing research surrounding embryonic development (Figure 3).
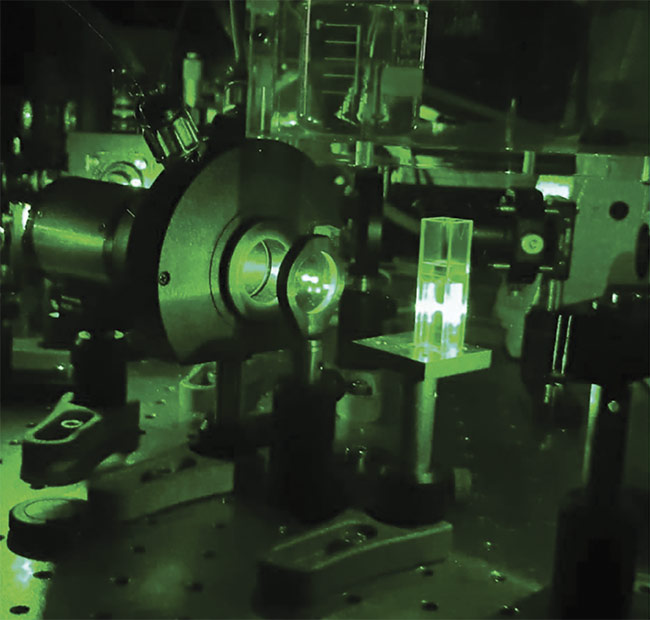
Figure 3. Close-up of a confocal Brillouin microscope, used by the Prevedel group at EMBL, focused on a glass cuvette filled with water. Courtesy of EMBL.
Brillouin scattering was first described by Leon Brillouin over a century ago, when he discovered that light can be shone on a sample, and will interact with the sample’s inherent thermal vibrations, producing a pronounced effect on scattering and subtly changing its color. But this imaging technique is slow because it gathers information from one point at a time, and the prolonged exposure can be toxic to a sample. In the case of line-scanning Brillouin microscopy, data is acquired from multiple points in parallel.
In a recently published article in Nature Methods, the team explained that they used this effect to accomplish fast 3D imaging of mechanical properties in the cells of fruit flies, ascidians, and mouse embryos. They employed an inverted selective plane illumination microscope with a 780-nm diode laser that not only reduces phototoxicity (as it is in the NIR range) but also does not interfere with common fluorophore signals, and has a narrowband filter to achieve consistent spectral purity (Figure 4). Data could therefore be taken in physiologically pertinent timescales for the first time during such processes as morphogenesis in fruit flies as well as cellular multiplication during mouse embryo development. The results were analyzed with the aid of a GPU1.
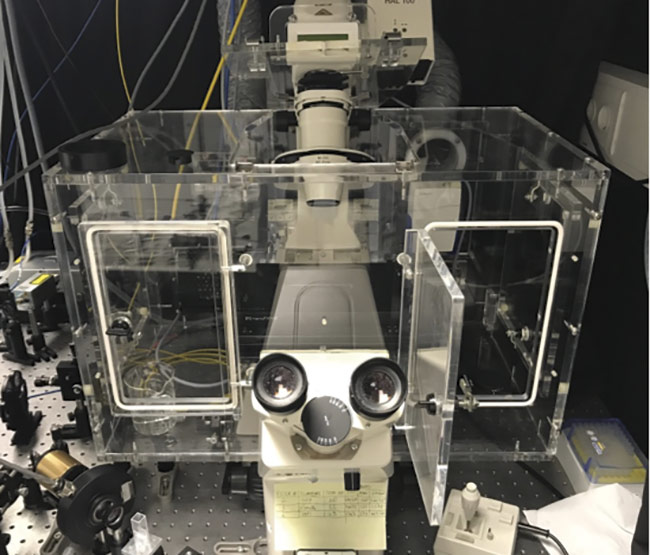
Figure 4. The top view of a confocal Brillouin microscope, which hosts an incubation chamber to maintain the sample environment. Courtesy
of EMBL.
“Embryonic development has previously been much studied from a molecular point of view, identifying important genes and signaling molecules and pathways involved in it,” said Robert Prevedel, group leader of the cell biology and biophysics unit at the EMBL. “However, the physical side (i.e., the forces and mechanical properties) that also plays an important role in this could so far not be measured with high spatial and temporal precision. Our technique makes measuring the mechanics of these processes now possible.”
Prevedel added that during dynamic cellular changes in any species, there can be very short-lived changes to the mechanical properties of biomaterials, and learning how or why this happens in space and time could answer some fundamental questions in biology. It could also show whether a new drug treatment is addressing what it needs to address.
“If you look at cancer detection, this is usually diagnosed based on manual palpation to feel changes in tissue stiffness,” he said. “But by the time one can feel it by one’s hands, millions of cells are already involved. We can track abnormalities at the single cell level, which would in principle reveal a condition much sooner.”
Flexible instrumentation
The companies currently offering systems usable for live-cell imaging have been faced with the challenge of enabling powerful imaging while maintaining the ability to adapt to the needs of a particular experiment or sample. And when it comes to measuring microscopic details, AI plays a role in dividing the picture into objects of interest and displaying how they relate to each other in a sample.
Leica Microsystems has sought to design systems that can be flexible for a wide variety of life sciences research, incorporating a number of different optical technologies and illumination mechanisms critical for unlocking information from live cells. Among them are the modular DMi8 inverted microscope, which is equipped with an advanced sequencer that dramatically reduces the lag between components, ranging from multiple cameras to filter wheels to motorized staging, with adaptive focus that can be adjusted to changing conditions.
“The advantage of an integrated and automated microscope system stems from the fact that one can automate multidimensional imaging (space/time/wavelength, etc.) even in a single imaging modality, independent of confocal/widefield or any other application needs,” said Abdullah Ahmed, global business excellence manager for life sciences research at Leica.
Such systems also use an AI-powered pixel classifier that enables swift image segmentation.
The company also offers a confocal microscopy platform called STELLARIS, which uses sophisticated detectors that capture a wide portion of the spectrum, operating with sensitivity and an extensive dynamic range, a white light laser, and accompanying analysis software. The platform is designed to provide a high level of contrast, and it captures lifetime information, which aids in research of cancer origins and progression, and the ever-evolving field of neuroscience.
Susanne Liebe, also a global business excellence manager for life sciences, said a primary motivator in system development was optimizing excitation and absorption.
“These microscopes are based on a concept of fully flexible excitation wavelengths and emission detection windows,” Liebe said. “This enables you to place an excitation line as close as possible to the absorption maximum of any given probe and to have the widest available detection window (410-850 nm). This combination maximizes signal generation (photons) as well as photon collection. Moreover, the spectral properties of fluorophores in live cells, tissues, and organisms may differ from those observed when free in solution. The ability to shift excitation or detection windows allows the user to take into account any such changes.”
Integrating technologies
In the case of the BrightEyes-TTM (time tagging module), the technology is rooted in fluorescence laser scanning microscopy. The module is part of the BrightEyes project, funded by the European Research Council, aimed at adapting confocal laser scanning microscopy for live-cell imaging and spectroscopy applications. The project centers around an asynchronous readout single-photon avalanche diode (SPAD) array detector capable of capturing the fluorescence signal from the sample in a photon-resolved manner2.
To record data, the SPAD array detector is combined with a time-tagging data acquisition module, the BrightEyes-TTM. This module is built around a field-programmable gate array (FPGA), which not only captures the position of the photon on the SPAD array but also records its arrival times concerning specific reference signals originating from the laser illuminating the sample and the optical components of the microscope. The module also offers a range of I/O connectors to interface with the microscope, the SPAD array detector, and the computer. Dedicated software running on the computer allows real-time data and image visualization.
In an article published in Nature Communications, researchers from the Italian Institute of Technology detailed the BrightEyes-TTM, which is connected to a protocol for integrating the module into a single-photon laser scanning microscope2.
By recording multiple (up to 49, the number of elements in the SPAD array) and simultaneous single-photon fluorescent events together with their highly precise (~30 ps) arrival times concerning the excitation events (synchronized with the pulsed laser), the operating software allows the reconstruction of a so-called time-correlated single-photon histogram of photon-arrival time, enabling the estimation of fluorescence lifetime.
Importantly, this system, compatible with many common commercial microscopes, facilitates measurements with reduced excitation light doses, thereby minimizing the risk of photodamage in live-cell experiments.
Giuseppe Vicidomini, a senior researcher at the Italian Institute of Technology, said this new construct introduces the potential to provide spatial and temporal information about samples at the single photon level, an aspect previously unattainable through conventional microscopy.
“In traditional fluorescence laser scanning microscopy, the fluorescence signal collected from a specific focal point on the sample is integrated over time and space by a photomultiplier,” Vicidomini said. “However, with our SPAD array detector and time-tagging module, we’re introducing a groundbreaking innovation that we’ve dubbed ‘single-photon microscopy.’ This approach no longer integrates photons across the space and time; instead, it captures the signal photon by photon. This provides essential spatial and temporal information for high-resolution imaging. For instance, we employ single-photon microscopy to examine intricate structures like protein and RNA condensates and aggregations. These insights play a pivotal role in unraveling the mysteries of numerous neurodegenerative diseases and pave the way for RNA therapeutics.”
Several institutions, such as Politecnico of Milan, and companies such as Genoa Instruments, collaborated on system components and design.
But live-cell imaging has already been embraced globally, as the aforementioned market report suggests. And the mission of scientists has evolved from why they should use it to how to maximize its impact and avoid pitfalls in implementation.
A recent article in Biophysics Reviews noted that the measurement of fluorescence and other features, the integration of numerous technologies into a setup, and strategies such as image segmentation and algorithm development, are all part of a user’s ultimate decision. The implementation of best practices, the authors wrote, will ensure that the results of live-cell imaging methods will be not only meaningful but also reproducible3.
References
1. C. Bevilacqua et al. (2023). High-resolution line-scan Brillouin microscopy for live imaging of mechanical properties during embryo development. Nat Methods, Vol. 20, No. 5, pp. 755-760.
2. A. Rossetta et al. (2022). The BrightEyes-TTM as an open-source time-tagging module for democratising single-photon microscopy. Nat Commun, Vol. 13, No. 1, p. 7406.
3. A.P. Cuny et al. (2022). Live cell microscopy: From image to insight. Biophys Rev, Vol. 3, p. 021302.
Checking the light source
AMSBIO, a transatlantic company, is actively harnessing lentiviral technology to achieve live-cell imaging that was previously unattainable with traditional fluorescence or bioluminescence. Lentiviruses are RNA-based viruses that provide a tool for gene delivery into cells. In the case of AMSBIO’s Nano-Lantern technology, a luminescent enzyme called Renilla-Luciferase is linked to an orange fluorescence protein.
Both traditional fluorescence and bioluminescence have their disadvantages when applied to imaging: Fluorescence requires external laser excitation, which can cause photobleaching, and bioluminescence provides inherently low brightness, requiring extended exposure times and limiting the ability to capture fast-moving processes. By contrast, the Nano-Lantern technology exploits bioluminescence energy transfer, wherein energy is transferred from a donor to an acceptor molecule, providing excitation and fluorescence bound to a protein, enabling faster and more accurate live-cell imaging.
The technology was initially developed in the laboratory of Professor Takeharu Nagai at Osaka University in Japan. A number of innovations at the Osaka lab have been used in the products released by AMSBIO.
“When supplied with the luciferase substrate, the luciferase transfers energy to the orange fluorescence protein, resulting in a fluorescent (luminescent) signal that peaks at 565 nm,” said Alex Sim, CEO of AMSBIO. “When these Nano-Lanterns are fused to a subcellular localization structure, a Bioprobe, or a target, the localized luminescence can be detected or the dynamics of the target can be tracked. Nano-Lantern technology does not require light activation and has extremely low-level background signal, making it more sensitive and quantitative.”
And Sim added that this light reaction is bright enough to be followed even at the single-molecule level. And it can thus be attached to specific biological processes, and the rapid movement of subcellular structures, in real time, with very little background noise (accompanying image). This capability has implications for research such as drug screening and single-cell tracking within animals and plants.
Sim said work is ongoing to integrate these innovations into other applications as well.
“The fantastic aspect is the ease of use,” he said. “Simply add the lentivirus to a mammalian cell culture and you have a user-friendly approach to live-cell imaging, enabling you to overcome the limitations of traditional fluorescence and bioluminescence techniques and providing valuable insights into cellular processes and dynamics.”
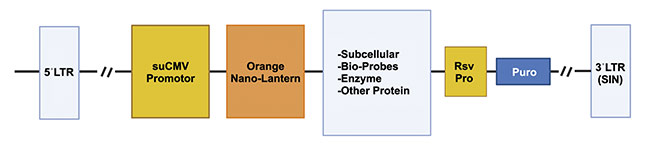
An illustration of the Nano-Lantern bio-probe scheme. Courtesy of AMSBIO.