Optical stimulation offers advantages over electrical stimulation.
by Jonathon Wells, Anita Mahadevan-Jansen, Chris Kao and E. Duco Jansen, Vanderbilt University; Mark Bendett, Jim Webb and Heather Ralph, Aculight Corp.; and Peter Konrad, Vanderbilt University Medical Center
Neural tissue stimulation is used in research and clinical applications. Neuroscientists
use nerve stimulation to answer fundamental questions about the function of the
nervous system and to research diseases such as Parkinson’s and Alzheimer’s
as well as biological processes such as nerve regeneration. Clinically, neural tissue
stimulation is used for applications including pain and depression management, control
of tremors and seizures, brain mapping and guidance of surgical resection. Although electrical stimulation has traditionally served as the standard method
to interface with neural tissue, researchers at Vanderbilt University in Nashville,
Tenn., have developed an optical technique called transient optical nerve stimulation
— a noncontact approach to neural activation.
Electrical stimulation has several fundamental
limitations. It requires physical contact with a metal electrode, often pierced
into the tissue, which can cause tissue damage. Spatial precision of stimulation
is often limited by the size of the electrodes and,
more importantly,by the inherent induction of an electric field, which initiates
a population response by recruiting multiple axons.1
Many neural stimulation applications require the
stimulus to be precisely within a small target area of tissue. Electrodes designed
to deliver precise stimulation have inherently high impedance characteristics, which,
in turn, impose higher voltage requirements to deliver the same charge, as dictated
by Ohm’s law. Electrophysiological recordings performed
to observe the response to electrical stimulation are plagued with the presence
of an inescapable “stimulation artifact” — especially when recording
in the vicinity of stimulation. The result is a recorded signal that contains a
stimulus-induced artifact that is typically much larger than the recorded action
potential and that requires significant signal processing to tease out the intended
signal.2,3 These limitations have driven researchers to pursue other means for neural
stimulation, including magnetic, ultrasound and other mechanical methods.
Transient optical nerve stimulation is a technique
that optically stimulates neural tissue using mid-infrared light. The method relies
on direct but transient (non-contact, pulsed) irradiation of the nerve surface with
an IR laser. The laser uses an optimized radiant exposure and wavelength to generate
(compound) action potentials and subsequent physiological effects; for example,
muscle contraction or sensory response.
The response is spatially precise,
permitting selective targeting of individual nerve fascicles with no observed tissue
damage.4,5 Moreover, optically induced action potentials exhibit no stimulation
artifacts, allowing adjacent stimulation and recording from a single site.
Optical stimulation is the direct induction
of an evoked potential in response to a transient targeted deposition of optical
energy. Only a pulsed source can be used for stimulation of neural tissue, and continuous-wave
irradiation will not lead to compound action-potential generation. Transient optical
nerve stimulation is fundamentally different from other biological light interactions,
such as biostimulation or low-level light therapy, where low fluence levels at
laser wavelengths that are weakly absorbed in tissue are applied continuously for
several minutes to elucidate some biological effect: use of light to activate caged
compounds or phototransduction in visual cortex mapping; or modulation of the excitability
of nerves using light, where light is used to alter spontaneous or stimulated neural
signals or potentials rather than being the primary source inducing that signal.
Much of the preliminary work in optimizing this
technique was performed in the peripheral nerve of rats in vivo. Theoretically,
the most appropriate wavelengths for stimulation depend on the architecture of the
target tissue. A typical rat sciatic nerve is approximately 1.5 mm in diameter and
consists of numerous nerve fibers grouped together in fascicles, each of which typically
innervates a specific muscle group. The number of fascicles per nerve varies greatly
across mammalian species, albeit the typical fascicle thickness is constant and
tends to be between 100 and 400 μm (Figure 1).6 Thus, the penetration depth
of the laser light must be greater than the distance from the nerve surface to within
the fascicle (~300 to 600 μm).
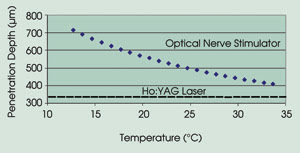
Figure 1. For optical stimulation, laser light must penetrate about 300 to 600 μm.
Careful wavelength tuning in the near-
to mid-IR (>1400 nm), where the main absorber is the water in tissue, allows
tunability of the optical penetration depth over several orders of magnitude.
The researchers identified 2.1 and
4.0 μm as the optimal stimulation wavelengths for the peripheral nerve —
those with maximum stimulation efficacy and minimum damage threshold. These wavelengths
correspond to valleys in tissue absorption and have nearly equivalent absorption coefficients. Stated differently,
the results showed that the most appropriate wavelengths for stimulation of the
sciatic nerve are those where the optical penetration depth is matched to the target
geometry; i.e., 2.12 μm has an optical penetration depth of 300 to 500 μm,
which corresponds to the depth and size of one fascicle.
Although there are few lasers that emit light
at 4.0 μm, and fiber optic delivery at this wavelength is problematic, the
Ho:YAG laser at 2.12 μm is commercially available and is currently used for
a variety of clinical applications. The scientists used it successfully for neural
stimulation with an average stimulation threshold radiant
exposure of 0.32 J/cm2 and an associated ablation threshold of 2.0 J/cm2. They compared
compound muscle-action potential and compound nerve-action potential traces from
electrical and optical stimulations of a rat sciatic nerve (Figure 2). This demonstrated
that the laser pulse can induce action potentials that are similar in shape and
timing to electrically evoked potentials, with greater spatial selectivity —
as indicated by fewer recruited axons and a lower amplitude — and without
artifacts.
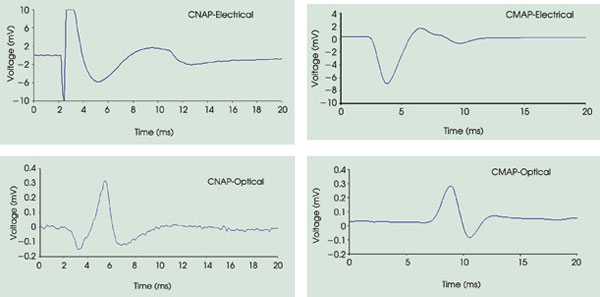
Figure 2. These graphs show compound nerve-action potential (CNAP)
and compound muscle-action potential (CMAP) recordings from transient optical nerve
stimulation with the infrared neural stimulator (0.4 J/cm2 and 2.7 ms) compared
with those from electrical stimulation (0.7 V) at the same site. Note that the electrical
stimulation masks the first 2 to 3 ms of the recorded nerve and muscle potential.
Stimulation occurs at 2 ms.
Spatial selectivity
Electrical
stimulation has an unconfined spread of charge radiating from the electrode. In
the case of peripheral nerve stimulation, as the injected current required for stimulation
increases, the volume of tissue affected by the electric field increases proportionally.
As the energy applied increases, more fibers are recruited, resulting in larger-amplitude
compound potentials. Thus, the compound nerve-action potential and compound muscle-action
potential represent a population response to electrical stimulation, made up of
individual all-or-none responses from many constituent axons, with a linear relationship between stimulation intensity and strength of the compound nerve-action potential response.7,8
Optical stimulation can be more spatially
precise than electrical stimulation. The limited optical penetration depth, small
spot size and lack of radial diffusion in tissue allow more selective excitation
of fascicles, resulting in targeted muscle contraction. A demonstration of the spatial
localization innate to optical stimulation is shown in Figure 3. Electrical stimulation
excites the entire nerve and elicits a subsequent twitch response from all innervated
muscles.
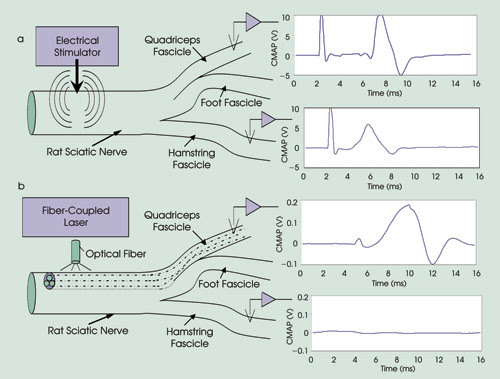
Figure 3. Compound muscle-action potential recordings from electrical
and optical stimulation were compared within the rat sciatic nerve using threshold
energies for each modality. The researchers placed recording electrodes within the
gastrocnemius and biceps femoris downstream muscles, approximately 40 and 55 mm
from the site of stimulation, respectively. Electrical stimulation with threshold
energy of 1.02 A/cm2 was delivered proximal to the first nerve branch point on the
fascicle leading to the gastrocnemius, and the muscular responses within gastrocnemius
and biceps femoris were simultaneously recorded. Using the minimum energy required
to stimulate contraction of the gastrocnemius still resulted in stimulation of the
neighboring biceps femoris fascicle, causing biceps femoris contraction (a). Laser
stimulation at a threshold of 0.4 J/cm2 resulted in a potential that is more than
an order of magnitude lower than that recorded with electrical stimulation in the
gastrocnemius with no response observed in the biceps femoris (b). Stimulation occurs
at 2 ms.
Optical stimulation causes a contraction
of the muscle innervated by the targeted nerve fascicle. Moving the laser spot across
the nerve targets individual muscle groups, demonstrating the selective recruitment
of nerve fibers with optical stimulation.
Before any technique can be applied
in living tissue, it is necessary to assess its safety and its limits. Toward this
goal, the researchers have carried out extensive studies to determine if transient
optical nerve stimulation might result in laser-induced damage to the stimulated
tissue.5 They observed no neurological functional deficit in survival studies after
stimulation at radiant exposures up to twice those needed to induce nerve stimulation.
Histological analysis of acute and survival (three- to five-day) experiments confirmed
these findings. Moreover, they believe that the stimulation thresholds that they
have found to date represent a worst-case scenario because of the extreme endpoint
of visible muscle contraction used in the experiments.
To extend the application of transient
optical nerve stimulation, the investigators teamed with Aculight Corp. in Bothell,
Wash., to develop a small, portable and low-cost optical stimulator called the infrared
neural stimulator. Based on the results of their wavelength optimization studies,
they determined that a laser wavelength with an optical penetration depth of 300
to 600 μm is required to stimulate the peripheral nervous system.
Although much of the research used the Ho:YAG
laser to provide this penetration depth, a careful look at the water-absorption
curve revealed that a diode laser with a wavelength of ~1.87 μm results
in the same penetration depth. Aculight had developed this device under a government
contract to produce nonstandard-wavelength lasers. A prototype laser for initial
studies required only relatively minor customization for the application.
A goal of the collaboration was to
develop an optical stimulator that would be affordable and rugged enough for experimental
or clinical use. The team chose diode technology for its simplicity, stability and
cost-effectiveness. Diodes emitting near 1.85 μm form the basis of the device.
In the commercial system, a single integrated housing contains the fiber-coupled
diode and all requisite control electronics. As with electrical nerve stimulators,
the control electronics in this unit enable manipulation and display of pulse energy,
width and repetition rate. However, a unique feature is the ability to vary the
emission wavelength, which allows adjustment of the tissue penetration depth. If
required, an external pulse driver and trigger interface are available to allow
the user to create pulse formats. The unit measures 13.25 x 12.5 x 4.74 in. and
weighs 11 lb.
The investigators tested the commercial
prototype system at the Vanderbilt laboratory during nerve stimulation experiments
similar to those of the initial research. The diode laser behaved similarly to the
Ho:YAG, affirming that the wavelength selection and the stimulation threshold were
similar as well. It provided safe, noncontact artifact-free peripheral nerve stimulation
using optimized laser parameters — yet with the simplicity, reliability and
ease of use of a diode-based system.
Although the studies showed that optical
stimulation is an effective and advantageous method for stimulation of neural tissue,
the obvious and intriguing question of the underlying
mechanism remains largely unanswered. Exactly what biophysical stimulus does the
absorbed laser light induce in the tissue that ultimately results in an action potential?
And, given this stimulus, what is the biological mechanism responsible for the transduction
into action potentials?
To a large extent, unraveling these mechanisms
is still in its infancy. At present, the scientists have strong evidence that the
underlying biophysical mechanism requires a temperature gradient, either spatial
or temporal. However, exactly how this is transduced into a functional action potential
remains an open question.
Future directions
The transient optical nerve stimulation project
is moving forward on several fronts. In addition to working on unraveling the underlying
mechanisms of optical stimulation, the researchers are proceeding toward demonstrating
stimulation in cells and tissues of the central nervous system. After the launch
of the commercial stimulator, they plan to develop specific clinical peripheral
nerve stimulators based on input from the nerve stimulation research community and
on further market research.
They also expect to address clinical
applications and stimulation of the central nervous system. This technology would
enable many as yet unrealized applications, including real-time multiplexed cortical
surface mapping for clinical and research purposes. The initial focus in the commercialization
of this device will be for a research-based infrared neural stimulator for use in
in vitro neurophysiological experiments as well as in in vivo animal studies.
In the long term, the collaborators
plan to pursue the clinical implementation of this device for peripheral and central
nervous system procedures. The long-range plan will focus on implantable devices,
potentially including vagus nerve stimulators, cochlear implants and other nerve
stimulation devices.
Meet the authors
Jonathon Wells is a PhD candidate in the department
of biomedical engineering; Anita Mahadevan-Jansen, an associate professor of biomedical
engineering; Chris Kao, an assistant professor of neurosurgery; and E. Duco Jansen,
an associate professor of biomedical engineering and neurosurgery, all at Vanderbilt
University in Nashville, Tenn; e-mail: [email protected].
Mark Bendett is the director of medical
projects; Jim Webb, a project manager; and Heather Ralph, development engineer,
all at Aculight Corp. in Bothell, Wash.; e-mail: [email protected].
Peter Konrad is associate professor
of neurosurgery and director of functional neurosurgery at Vanderbilt University
Medical Center in Nashville, Tenn., e-mail: [email protected]u.
References
1. D. Palanker et al (2005). Design of a high-resolution optoelectronic retinal prosthesis. J NEURAL ENG, Vol. 2, pp. S105-120.
2. C. Miller et al (2000). An improved method of reducing stimulus artifact in the electrically evoked whole-nerve potential. EAR HEAR, Vol. 21, pp. 280-290.
3. K. McGill et al (1982). On the nature and elimination of stimulus artifact in nerve signals evoked and recorded using surface electrodes. IEEE TRANS BIOMED ENG, Vol. 29, pp. 129-137.
4. J.D. Wells et al (2005). Optical stimulation of neural tissue in vivo. OPT LETT, Vol. 30, pp. 504-507.
5. J.D. Wells et al (2005). Application of infrared light for in vivo neural stimulation. JOURNAL OF BIOMEDICAL OPTICS, Vol. 10, 064003.
6. G. Paxinos (2004). The Rat Nervous System. Third edition. Elsevier.
7. L.A. Geddes et al (1985). Tissue stimulation: theoretical considerations and practical applications.
MED BIOL ENG COMPUT, Vol. 23, pp. 131-137.
8. L.A. Geddes and J.D. Bourland (1985). The strength-duration curve. IEEE TRANS BIOMED ENG,
Vol. 32, pp. 458-459.
Optical Stimulation of Auditory Neurons
Dr. Claus-Peter Richter, Northwestern University
One of the most
promising uses for optical stimulation appears to be in the area of cochlear implants.
Cochlear prostheses bypass damaged hair cells in the auditory system by direct electrical
stimulation of the auditory nerve. Multiple-electrode cochlear implants are designed
to stimulate discrete spiral ganglion cell populations along the cochlea.
However, discrete neural populations
cannot always be electrically activated. In fact, with closely spaced electrode
pairs at high current levels, a broad region of auditory neurons is activated. When
this occurs, sound sensation may be confused or indistinguishable, reducing the
number of independent channels of information that can be conveyed to the cochlear
implant user.
Research at Northwestern University
in Evanston, Ill., is focused on the design of cochlear implants that stimulate
smaller populations of spiral ganglion cells. We are finding that optical stimulation
is an effective technique for neural interfaces. Its advantages over electrical
stimulation are its spatial selectivity and its noninvasive character.
We have demonstrated in a gerbil that
extremely small populations of cochlear spiral ganglion cells can be optically stimulated
over extended periods of time. Our first experiments, made with a Ho:YAG laser with
a 2.12-μm wavelength and a 250-μs pulse duration, demonstrated that optical
radiation evokes electrical responses from the auditory nerve in animals with normal
hearing and in those that are deaf. Laser radiation could be increased by 30 to
40 dB until drastic changes were seen in cochlear function. Cochlear responses to
optical radiation were stable over extended stimulation times.
More recent experiments with a fiber-coupled
diode laser from Aculight Corp. of Bothell, Wash., employed stimulation rates up
to 1 kHz. The experiments showed that single auditory nerve fibers could follow
light-pulse repetition rates up to around 300 pulses per second and that such rates
did not result in obvious neural tissue damage.
With Aculight’s recent development
of smaller stimulation units, we plan to chronically implant the devices in an animal
model. In the future, we hope to develop laser-based cochlear implants that allow
parallel processing of information on many channels.