Pulsed lasers on a porous sheet allow for efficient microalgae production on a scale that can answer the need for cultivating a source of clean energy.
Dimitris Alexandropoulos and Konstantina Tourlouki, University of Patras; and Simone Mazzucato, SISMA SpA
Ever-increasing environmental concerns have placed biofuels in the spotlight of research and development efforts in search of a clean energy solution. In this context, microalgae biomass harvested with pulsed lasers is a viable alternative for the production of biofuels, including biodiesel, bioethanol, biogas, and biohydrogen1.
Microalgae are rich in energy content and their mass production makes techno-economic sense. In addition, they offer an inflated growth rate, a substantial capacity for CO2 fixation, and the release of O2 into the environment2. Microalgae’s main competitors for alternative sources of biodiesel production are conventional crop plants, yet microalgae are expected to outperform these by a factor of 15 to 300 in oil production3. Microalgae can be harvested every 14 days (depending on the species and cultivation method) due to their quick growth rate, whereas conventional crop plants are harvested just twice per year. Additionally, microalgae production of biofuel is greener than crop plant cultivation because its process requires less water consumption.

Microalgae is cultivated in the laboratory. Adapted from Reference 6. Courtesy of CC BY 4.0.
Despite the advantages of microalgae biofuel production over other alternative fuel production methods (in theory), the practice is currently limited by expensive and inefficient harvesting methods. Harvesting amounts to approximately 20% to 30% of the total cost of cultivation4, and, therefore, there is a big margin for improvement in financial viability.
A wide range of microalgae harvest-ing methods have already been developed, including centrifugation, flocculation, magnetic separation, and coagulation5. The disadvantages of these techniques are the overall operational costs (€0.5 to €2 per kilogram for dry weight algae) and the high energy demand (ranging from 0.2 to 5 kWh/kg).
In a recently published work6, the authors address the challenge for an efficient, low-cost collection of microalgae’s biomass by developing a new harvesting method based on laser-processed glass-fiber-reinforced polymer (GFRP) (Figure 1).
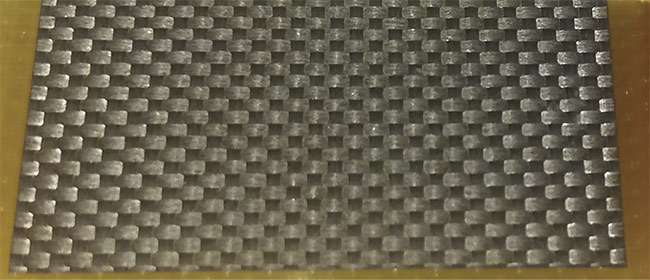
Figure 1. A laser micromachined glass-fiber-reinforced polymer (GFRP). Adapted from Reference 6. Courtesy of CC BY 4.0.
This approach mimics honey harvesting in honeybee combs. The GFRP panels are processed to reveal the glass fiber mesh (with diameters of ~10 μm), which serves as the adhesion layer for the microalgae development. This design is based on the observation that microalgae preferentially develop on microporous surfaces. This makes the revealed mesh glass fiber of GFRP ideal for the cultivation of microalgae. Much like honey harvesting, microalgae can be extracted from the panels using techniques that are industrially compatible, such as with a blade or a centrifugation-based extractor. Additionally, the use of GFRP panels as cultivation surfaces is compatible with vertical bioreactors, which create homogenous mixing and are used for the maximization of microalgae productivity.
The main technologies at use in the new microalgae cultivation method are GFRP and laser processing. GFRP is a common composite material that is relatively inexpensive and readily available. It is widely used in the marine industry, as well as in aerospace as a light aircraft construction material. The laser used for the surface processing of GFRPs is an industrial UV laser inherently suitable for large-scale industrial production.
For the purposes of the experiment, a commercially available bidirectional GFRP sheet was used. The surface of the GFRP was processed with a SISMA laser station that comprises a 15-W Q-switched UV laser emitting at 355 nm and operating in the nanosecond regime, a galvanometer scanner, and an
f-Theta lens to direct and focus the beam. The wavelength of 355 nm was chosen because it removes the GFRP resin, leaving the glass fiber network intact. The glass network is porous and rough, thus favoring microalgae adhesion and the initiation of the cultivation process. Also, the glass fibers are transparent to allow in much needed light for the microalgae cultivation. The microscopic photos of the UV laser micromachined GFRP samples are shown in Figure 2.
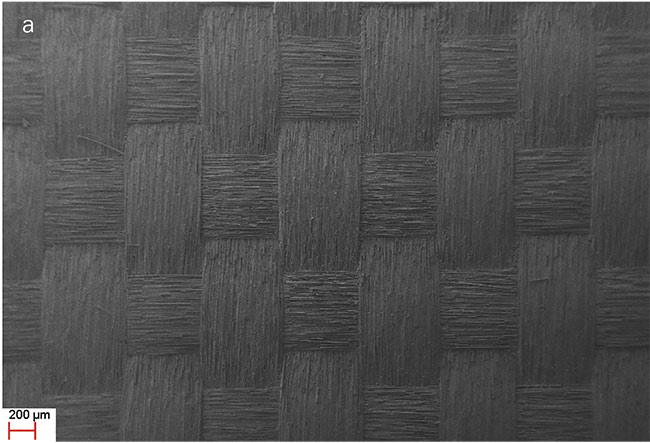
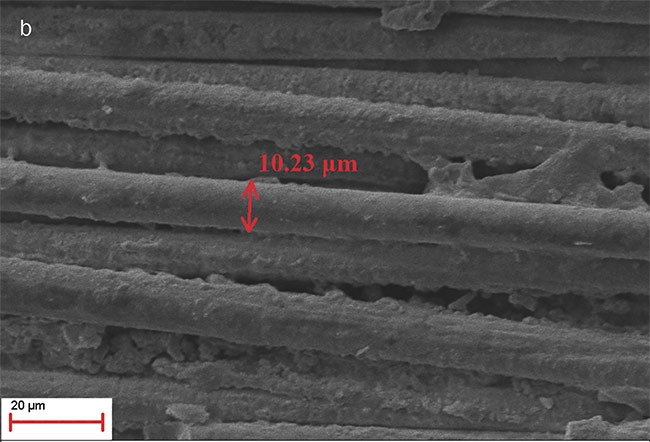
Figure 2. Scanning electron micros-copy image. GFRP area after UV laser micromachining (a). The resin has been removed, revealing the inner glass fiber mesh (magnification: 61×). Glass fiber thickness: 10.23 μm (magnification: 2.09K×) (b). Adapted from Reference 6. Courtesy of CC BY 4.0.
The harvesting method was analyzed by growing the microalga Scenedesmus rubescens because it can be found in fresh water and wastewater and can be used for biofuel production. The glass fiber thickness was 10.23 μm, comparable to the Scenedesmus rubescens’ cell size.
An open pond system was developed that consisted of three rectangular reactor vessels for the cultivation of the alga (Figure 3). The laser-processed GFRP, in the form of coupons, were placed in the pond system; the culture was left to grow exponentially for 16 days; and the coupons were gradually removed every four days.

Figure 3. Scenedesmus rubescens microalgae cultivation system. Adapted from Reference 6. Courtesy of CC BY 4.0.
The collected GFRP coupons exhibited a microalgae biofilm formed on their surface. The growth of suspended algal biomass in the reactors was quantified by measuring the number of cells. Figure 4 shows the evolution of the measured algae biomass along with days of cultivation. Τhe maximum biomass recovery (16-day cultivation) from GFRP coupons was 13.54 g/m2, corresponding to
0.84 g/m2/d.
In the literature, algal biofilm productivities reach values up to 6.3 g/m2/d, depending on reactor type and the substrate used7. The potential for even greater microalgae cultivation exists, since there are no signs of growth saturation within the time span under study. The greater the algae mass that is produced, the greater amount of lipids — and, therefore, biofuel — that is generated.
Figure 4 also shows pictures of the GFRP coupons taken on various days. It is evident that microalgae first develop on the exposed glass fiber mesh. Toward the end of the experiment (day 16) microalgae begin to develop on the untreated resin as well. These results are promising and support the applicability of the proposed harvesting method. It is believed that the algae biomass developed on GFRP can be increased if the roughness of the glass fiber mesh is enhanced. Then fiber mesh discontinuities, in the form of broken fibers, would act as anchor points for the microalgae organisms, resulting in the creation of thicker biofilm. Given that UV laser radiation leaves the fiber intact, laser processing at other wavelengths must be sought to break them.
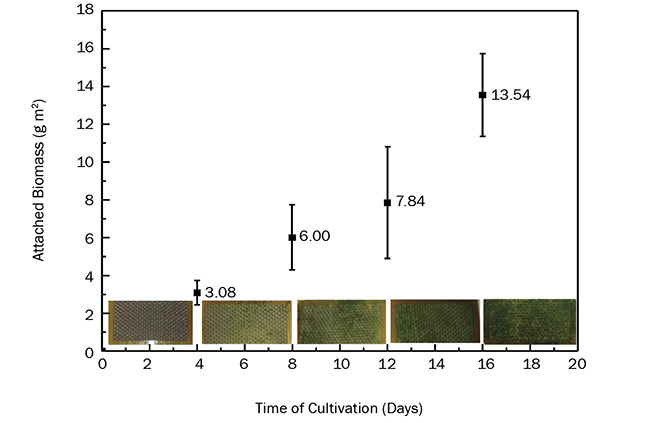
Figure 4. Algae biomass adhesion on UV laser micromachined GFRP over time. The graph includes the mean value of the algal biomass that attached on two replicate GFRP coupons. The coupons, collected from the cultivation every four days, can be seen at the bottom of the graph. Adapted from Reference 6. Courtesy of CC BY 4.0.
An added advantage of this method is that GFRP harvesting panels can be adjusted to the bioreactor geometry, including vertical positioning in a cultivation pond, for maximum light utilization and algae colonization. Additionally, the GFRP surfaces can be reused many times as harvesting panels, further reducing the overall harvesting cost.
Laser-processed GFRP surfaces have demonstrated potential for algal adhesion and thus can be used for harvesting. The proposed harvesting technique is simple and cost-effective and benefits from GFRP materials research. The GFRP harvesting panels are reusable and can be of any shape and size, providing flexibility and, most importantly, scalability.
Over the last decade, research has focused on finding ways to upscale and commercialize microalgae cultivation. Microalgae can grow in many different environments and make use of multiple types of water, from fresh water to wastewater. The preliminary results serve as a proof of concept for a harvesting method. It is anticipated that optimization of the described process will result in improved biomass recovery values comparable with other more complex and costly methods. This improves the techno-economics of the microalgae cultivation, not only for biofuel creation, but also for bioactive medicinal products and food ingredients — thus paving the way to large-scale industrial production.
Acknowledgment
Professor Ioannis D. Manariotis and Vasiliki Tsavatopoulou, from the Environmental Engineering Laboratory in the Department of Civil Engineering at the University of Patras, are acknowledged for developing the open pond system for the growth of the microalga Scenedesmus rubescens.
Meet the authors
Dimitris Alexandropoulos received a B.Sc. degree in physics in 1998 from the University of Athens in Greece, an M.Sc. degree in the physics of laser communications in 1999 from the University of Essex in England, and a doctoral degree in 2003. He is now an assistant professor in nanophotonics in the Department of Materials Science at the University of Patras in Greece. Alexandropoulos is also a visiting fellow of the School of Computer Science and Electronic Engineering at Essex. Since September 2018, he has been an affiliated member at the Industrial Systems Institute of the Athena Research Center in Greece. His recent research activities focus on photonic materials, fabrication of micro/nanophotonic structures and
holographic optical elements, laser micromachining and applications, and VCSELs and integrated optoelectronic circuits; email: [email protected].
Konstantina Tourlouki works as a photonics engineer at Nanotypos in Greece. She is a graduate student in applied optoelectronics, with an undergraduate degree from the Department of Materials Science at the University of Patras in Greece; email:
[email protected].
Simone Mazzucato works as a technology research specialist in the Laser Processing Division of SISMA SpA in Italy. He graduated in telecommunications engineering from the University of Padua in Italy, received a doctorate in electronic systems engineering from the University of Essex in England, and worked as an experimentalist for several years in various EU academic institutions. Mazzucato has co-authored more than 50 scientific journal and conference publications in the fields of physics, engineering, and physical chemistry, mainly in regard to material laser characterization; email: [email protected].
References
1. M.A. Hattab et al. (2015). Microalgae harvesting methods for industrial production of biodiesel: critical review and comparative analysis. J Fundam Renew Energy Appl, Vol. 5.
2. K. Dincer et al. (2008). Lower emissions from biodiesel combustion. Energy Sources Part A, Vol. 30, pp. 963-968.
3. F. Alam et al. (2012). Biofuel from algae — is it a viable alternative? Procedia Eng,
Vol. 49, pp. 221-227.
4. E. Molina Grima et al. (2003). Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv, Vol. 20, pp. 491-515.
5. A.I. Barros et al. (2015). Harvesting techniques applied to microalgae: a review. Renewable Sustainable Energy Rev,
Vol. 41, pp. 1489-1500.
6. K. Tourlouki et al. (2020). A novel microalgae harvesting method using laser micromachined glass fiber reinforced
polymers. Photonics, Vol. 7, Issue 42, www.doi.org/10.3390/photonics7020042.
7. B. Podola et al. (2017). Porous substrate bioreactors: a paradigm shift in microalgal biotechnology? Trends Biotechnol, Vol. 35, pp. 121-132.