Lynn M. Savage
Assembling optical components requires some type of bonding mechanism, often using epoxies, glass frits or other materials to join two or more pieces. No matter what type of bonding material or process is used, however, there often are limitations to overcome, such as high temperature requirements, low reliability or strength of the bond, and subpar optical properties of the interface between the bond and the component.
Now researchers affiliated with Stanford University and with Lawrence Berkeley National Laboratory, both in California, have developed a novel bonding process that utilizes sol-gel chemistry techniques and silica nanoparticles to overcome many typical hurdles.
Steven Chu, the director of the lab, and his associate, Sanjeevi Sivasankar, based their process on the existing technique of hydroxide catalysis. In this process, an alkaline solution such as sodium silicate binds two surfaces by etching them and forming siloxane chains between them. According to Chu and Sivasankar, however, this form of bonding has low reproducibility and can be used only on components with high surface flatness and cleanliness.
In standard hydroxide catalysis, two surfaces are bonded by introducing them to a solution that hydroxylates them, causing short siloxane chains to protrude. If the surfaces are perfectly flat, a tight chemical bond forms. However, because most optical surfaces are not perfectly flat, something is needed to fill the gaps and to strengthen the bond.
The scientists suspended 5- and 7-nm silica particles in a KOH solution at concentrations of 15, 18 or 21 percent weight/volume. They placed the particles and bonding solution on soda-lime glass slides and monitored the curing process. As the interface dehydrated, the silica nanoparticles filled the gaps between the siloxane chains and polymerized into highly branched, three-dimensional networks.
The researchers found that, when the ratio of hydroxide to silica was 0.35, the soda-lime glass slides bonded successfully every time. “The chemistry of the nanoparticle-mediated bonding will work with any material that has silica or SiOH groups on its surface,” Sivasankar said.

They also found that increasing the initial pH or decreasing the nanoparticle concentration enabled them to lengthen the curing rate, which would aid optical alignment during the curing process. The bond strength increased from 0.2 MPa after 1 h to 8.5 MPa after 120 h to 14 MPa after 300 h and, on average, slides bonded with silica nanoparticles showed only a few small bonding defects, mostly along the edges (see figure).
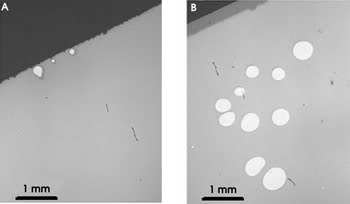
Bright-field microscopy images show soda-lime glass slides bonded using a nanoparticle-mediated technique (A) and a standard silicate bonding technique (B). Slides bonded using silica nanoparticles were nearly free of defects, with most of the existing defects appearing along the edges. Slides attached using the silicate bonding method had more — and larger — defects, although none along the edges. Reprinted with permission of the American Chemical Society.
According to Sivasankar, the method produces bonds that are thicker than optical contacting and direct sol-gel bonding, making it less suitable for optical systems with low mechanical dissipation, such as gravitational wave detectors.
Nano Letters, October 2007, pp. 3031-3034.