Lasers and sensors used in flow cytometers are making major strides — becoming smaller, more powerful, and more affordable. These advancements are helping improve clinical diagnostic tools used in detecting cancer cells.
PEDRO MUÑOZ AND ANN RUSSELL-EL DEMERY, OSRAM OPTO SEMICONDUCTORS
The outstanding achievements of immunologists James P. Allison and Tasuku Honjo — winners of the 2018 Nobel Prize for medicine for their discovery of cancer therapy by inhibition of negative immune regulation — are at the forefront of an active research field that will soon enable us to “educate” the immune system to fight specific cancer cells. The research could lead to a cure for many cancer forms, likely reducing the reliance on chemotherapy.
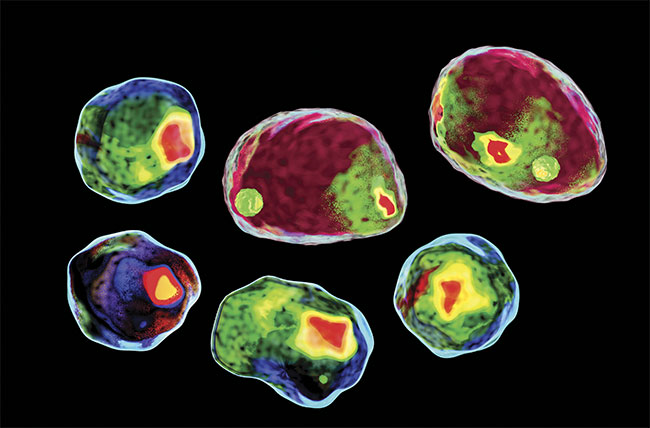
Such fantastic and inspiring prospects are pertinent to many other fields of medicine, well beyond cancer treatment. What many people don’t know is that most of these emerging therapies require the use of an innovative tool: the flow cytometer. Key components of flow cytometers are lasers and sensors, which have made tremendous advancements contributing to the major technological shift seen in clinical diagnostics. These photonics technologies are still evolving and will continue to influence the medical diagnostic market, especially regarding flow cytometry.
The flow cytometer is a ubiquitous and sophisticated tool used since the 1960s for cell counting and sorting and biomarker detecting. False positives and cell misidentification drove the technology to include imaging-in-flow techniques such as flying spot scanners, slit-imaging onto an array, strobing lasers, and mirror tracking, which are all used to prevent errors1. Polychromatic light allows measurement of cell size, fluorescence of attached biomarkers, and scatter, while the different ratio of forward and side light scatter serves to separate the cells into different populations such as monocytes and leukocytes2.
When fluorescent-marked cells pass through the laser beam, they are detected by a fluorescent readout that identifies a specific molecular feature of a cell and can be used to identify and count carcinogenic cells. This process can work only if the protein is a known apriori, which can be difficult. However, with the understanding of cancer cytology and the diagnostic power of a flow cytometer, some studies show that future cancer diagnostics may be accessible through the analysis of a blood sample, which could eliminate the need for biopsies of tissue in some cases.
A complex tool
Flow cytometers are complex optical tools that have benefited from miniaturized solid-state lasers and affordable CCD, CMOS, and p-i-n detectors. The previously used bulky gas ion lasers that operated with less than 1% efficiency required very high-powered drivers and extensive warm-up times. The solid-state lasers used today require much less power and space, and less complex optics, and they are altogether more compact and robust.
Implementation of several different wavelengths in a system has become easier, increasing the number of different dyes that can be used on a single sample. A typical benchtop flow cytometer often uses 405-, 488-, and 635-nm lasers. In an optical path, the stimulating laser light needs to be filtered to prevent flooding of the sensors so the relatively weak fluorescent readout can be detected. This process is achieved with narrow-bandwidth filters, dichroic mirrors, and beamsplitters. The lasers used require wavelengths of just a few nanometers, to match the filters and to increase the fluorescent response of the fluorochromes, thereby making LEDs a very poor choice of illumination.
One of the main drivers of the shift from gas lasers to solid-state lasers in flow cytometry was the technology used in CD players. In 1970, Bell Labs developed the first semiconductor laser diode that operated for long periods of time. The first CD player was offered by Sony in 1982 and used a similar laser diode. As CD player sales soared into the millions and the CD-ROM made its debut, laser diodes became affordable for medical tools as well.
Infrared diode lasers (808 nm) were also developed in large quantities and used in diode-pumped solid-state (DPSS) lasers — emitting at 1064 nm and frequency doubled to 532 nm — the most common laser type used in flow cytometry today. DPSS lasers provide a high-optical-power output with transversal single-mode beam quality, but they use a large number of optical components that need to be aligned (Figure 1), making them expensive to manufacture. Red lasers became available as DVD technology soared, but optimal wavelengths for flow cytometers were not available.
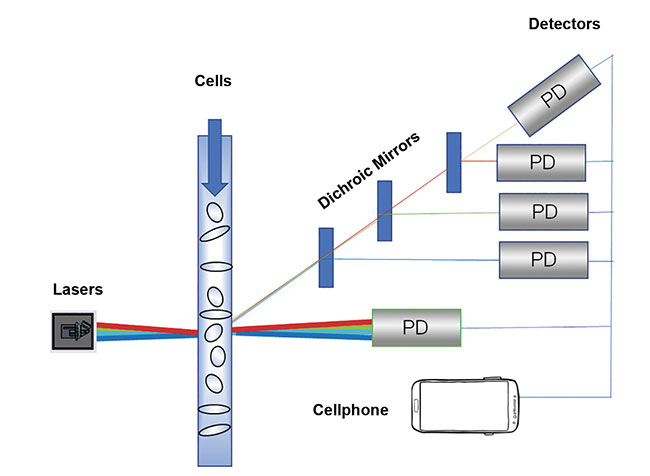
Figure 1. Older DPSS lasers used more than 20 individual components. Courtesy of Osram Opto
Semiconductors.
With the launch of the 405-nm laser created for Blu-ray technology, and the new direct-transition 520- and 488-nm laser diodes by Osram Opto Semicondutors, many of the laser wavelengths required to replace DPSS lasers in flow cytometers became available. Osram’s direct green laser diode was a breakthrough driven by the desire to incorporate full-color laser projectors into smartphones3. The company’s 520-nm system led to the development of the PLT5 488-nm cyan biomedical laser diode (Figure 2).
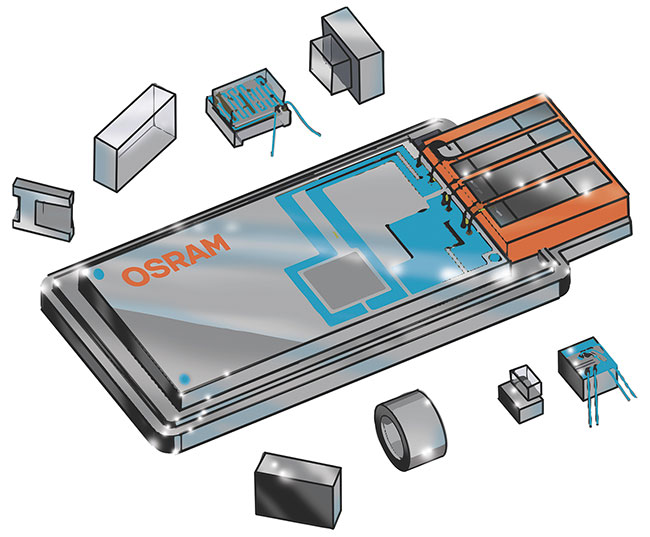
Figure 2. Flow cytometers, featuring lasers and detectors, are used for cell counting and sorting, and detecting biomarkers. Courtesy of Osram Opto Semiconductors.
The 488-nm wavelength is the most common in life science research applications because hundreds of fluorophores excite around the laser’s center wavelength. This laser allows for simple, cost-effective designs thanks to its far-field pattern and tight wavelength tolerance of ±2 nm.
Affordability developments
Further developments enabling affordable flow cytometry include new laser diode packaging technologies that improve manufacturing and package thermal resistance, as well as submillimeter proximity of various wavelength laser dies. The RGB lasers used as picoprojectors for head-up display and near-to-eye virtual and augmented reality require a tiny form factor.
These same technologies could be applied to medical devices, and could enable pocket-sized flow cytometers that could be delivered to a sick patient in a remote area, or used for continuous monitoring of patients in ambulances. These potentially life-saving devices would require a multiwavelength laser module that is integrated into a compact package.
To detect the forward signal, a large-area p-i-n photodiode is commonly chosen. This photodiode is a relatively simple component delivering a photocurrent based on the photoelectric effect on a p-n junction under a reverse voltage. New advancements driven by the need for miniaturization of head-mounted virtual and augmented reality devices include integrated dark current suppression, thermal compensation, and signal amplifiers, as well as built-in digital signal output conversion and voltage output within the same small (mm) packing size. A precursor of these new developments is the tiny ambient SFH 5711 light sensor. All of these features increase the signal-to-noise ratio and the accuracy of the reading.
Most flow cytometers use photomultiplier tubes for detecting the scattered light signal, because a much higher signal-to-noise ratio is required to detect very faint light scattered by single cells. In this part of the flow cytometer, substitutes for the bulky photomultiplier tubes include avalanche photodiodes (APDs), single-photon avalanche diodes (SPADs), and silicon photomultipliers (SiPMs). These semiconductor-based sensors are superior in performance and much more compact and robust than the photomultiplier tubes. Although APDs, SPADs, and SiPMs are lower in cost than the photomultiplier tubes, they are still relatively expensive because they are only found in low-volume, niche applications.
With advancements in lidar technology for autonomous vehicles (Figure 3) and robotics, which need powerful 3D range and depth sensing, the market for very sensitive detectors with low intrinsic noise and high photocurrents is growing. APDs and SiPMs are the most likely choices for 905-nm pulsed lasers to extend the sensing range of the lidar system because of their high intrinsic gain. The growing market for autonomous vehicles and the large number of sensors required for one vehicle will ultimately not only drive technology improvements, but also reduce cost. So autonomous driving technology could indirectly help cure cancer by driving down the price of flow cytometers through the replacement of photomultiplier tubes with newer sensors.

Figure 3. The most common laser diodes in life science research are 488 nm. Courtesy of Osram Opto Semiconductors.
These advancements are helping to make flow cytometry tools smaller, more powerful, and more affordable, leading to countless new research opportunities for scientists. The future of cancer therapy could include customized analysis
using flow cytometry to diagnose cancer stages and treat specific cancer types.
The heterogeneity of cancer cells and their antibody responses are substantial and the immune response is complex. In the future, flow cytometry use could occur at the point of care with a tabletop or portable tool. Small, semicomplex flow cytometry devices may also be possible, with a cloud data link and AI-driven suggestions for sample preparation and appropriate antigens and fluorochromes for optimized results.
Meaningfully interpreting the readings from a large amount of available laser excitation wavelengths can be challenging. Software solutions have become a large portion of the size of the flow cytometry market, which was estimated to be valued at $1.6 billion in 2016. Some studies estimate an increase to $8 billion by 2025. Despite these large market numbers, most of the actual tools are being used for research and development and not for point-of-care of patients.
The current advancements in medical research, as well as those in the diagnostic capabilities provided by flow cytometric technology, will provoke a shift in the flow cytometric business landscape. Instead of the technology being used only for research (with cumbersome, expensive tools), the market can be revolutionized by less complex tools that rely on cloud computing and AI to assist medical personnel in making diagnostic and therapeutic decisions for patients.
Meet the authors
Pedro Muñoz, Ph.D., is a product marketing manager at Osram Opto Semiconductors (North America), where he specializes in emitters, lasers, and sensors. He has a physics degree from the University of Vienna and
a doctorate in physics from the University
of Cologne in Germany; email: [email protected].
Ann Russell-El Demery, Ph.D., is a senior
application engineer at Osram Opto Semiconductors (North America), where she specializes in near-to-eye, RGB laser light engines.
She has a doctorate in electrical engineering from the University of Cincinnati; email: [email protected].
References
1. Y. Han et al. (2016). Review: imaging technologies for flow cytometry. Lab Chip,
Vol. 16, Issue 24, pp. 4639-4647.
2. S. Perfetto et al. (2004). Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol, Vol. 4, Issue 8, pp. 648-655.
3. Osram Opto Semiconductors (January 2019). Osram enables projections with the smartphone, www.osram.com/os/press/press-releases/osram-enables-projection-with-the-smartphone-new-green-laser-plt3_520d-to38.jsp.