The simplicity of fused silica’s base formula belies the range of its production methods, performance qualities, and grades.
BERNHARD FRANZ, HERAEUS CONAMIC
Many users of fused silica may ask why there are so many different grades of the glass. How much variety can you expect from the simple chemical formula SiO2 — and does this wide variety have any effect on real-world optics?
Despite fused silica’s deceptively simple formula, it can exhibit various characteristics based on the production processes and the raw materials employed, which makes it a very versatile optical material.
In general terms, fused silica has a very broad optical transmission window, ranging from the UV to the near-infrared (NIR). It is chemically stable even in harsh environments and can sustain higher temperatures than many optical coatings. Additionally, the local variation of the fused material’s refractive index, especially in larger optics, can be smaller than in aluminosilicate glasses.
But is there more to distinguish the possible varieties of fused silica than a dry fused option for NIR optics and a wet one for UV applications?
The main factors influencing key
parameters are production processes
and raw materials. The most obvious
correlation is between the purity of the raw fused silica material and fused silica’s spectral absorption, especially in the UV range.
Impurities, in the form of metallic ions or hydroxyl groups (OH) can cause greater absorption and influence the
viscosity of the glass.
Greater absorption leads to loss of transmission, higher signal noise, and heating of optical components, which, in turn, results in thermal lensing.
Impurities
There are two sources of metallic impurities in fused silica. The first is the raw material, and the second is the actual fusion process used to fabricate the glass. If vitreous silica is produced from natural raw materials, the metallic impurity content is typically, at best, in the parts per million range. For fused silica made of chemical precursors, the impurity level is in the parts per billion range — an improvement by a factor 1000.
Metallic ions are sometimes intentionally used as dopants to color SiO2 and instill absorbing properties. Such dopants are typically intended to affect transmission in the UV. Other than for filter glasses, however, metallic impurities are to be avoided in fused silica.
Metallic ions can become incorporated in the glass network comprising Si-O bonds in two ways. They either substitute for a silicon ion, or, failing to bind directly into the SiO2 network, they instead become surrounded by the matrix, similar to the interstitial ions in crystals (Figure 1).

Figure 1. The atomic SiO2 glass matrix with metallic impurities and hydroxyl (OH) groups. Courtesy of Heraeus.
While gaseous atoms have distinctive absorption lines, metallic impurities in glass exhibit line broadening defined by the atomic or electronic vicinity of the ion in the matrix.
Production processes
Today’s fused silica producers have optimized their fusion processes over several decades to yield the purest vitreous silica possible. The two most common processes used to produce vitreous silica are direct quartz and two-step vapor deposition.
In the direct quartz process, chemical
precursors are burned to produce nanoscale SiO2 particles that are continuously melted to form bulk fused silica.
In the vapor deposition process (vapor axial deposition), these nanoscale
particles are called soot (Figure 2). The soot is collected to form a macroscopic chalk-like soot body that can be dried to remove OH groups and, in a second step, to be vitrified at high temperatures to form a solid glass body.
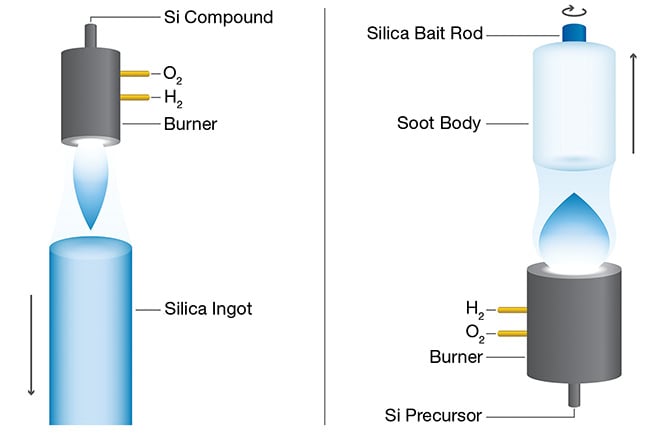
Figure 2. Today’s fused silica producers have optimized their fusion processes over several decades to yield the purest vitreous silica possible. The two most common processes that they use to produce vitreous silica are direct quartz (left) and vapor axial deposition (right). The vapor axial deposition process involves two steps — deposition and vitrification — that can modify the chemical structure of fused silica, while the direct quartz process directly produces a very OH-rich grade of the material. Courtesy of Heraeus.
Both fusion processes determine the content of metallic impurities in the bulk material and its OH content. The higher the OH content, the higher the material’s absorption in the NIR band, specifically around 1385 and 2210 nm, and in a broader band around 2720 nm (Figure 3).
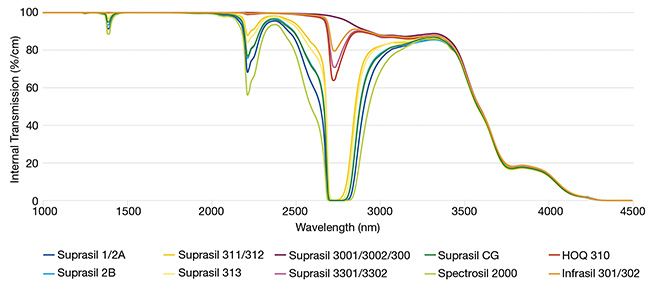
Figure 3. NIR transmission of fused silica grades measuring 10 mm thick. Transmission differs according to the grade’s OH content. Courtesy of Heraeus.
Typically, a high OH content is also often accompanied by dissolved molecular hydrogen (H2). This hydrogen passivates absorption centers generated under UV radiation and thereby prevents photodarkening, making H2 beneficial to the lifetime of fused silica components in UV-intensive applications. In applications in which residual H2 content is not enough to improve lifetime, the content can be enhanced by storing the glass blank in an H2-containing atmosphere.
There are drawbacks to high contents of OH and H2. Local fluctuations of these elements in fused silica are a source of variation in the
material’s refractive index — a quality commonly referred to as optical homogeneity.
Optical homogeneity is determined by local variations in the glass caused by the production process. The concentration of impurities, the OH and H2 contents, and the number of defect centers, as well as the temperature distribution during deposition, can all affect optical homogeneity.
A detailed analysis of optical
homogeneity can be important for some applications, and it is performed using Zernike polynomials, which are a set of orthogonal polynomials used to describe optical wavefront functions for an optical component or system. In such analysis, it is common to check for the peak-to-valley (P-V) homogeneity. But for more demanding applications, it is important to understand the contribution of the different higher-order terms or to even consider the residual fluctuations.
Generating blanks of fused silica with very low P-V variations typically requires their selection from a larger piece of fused silica. However, despite the low P-V value of these blanks, higher-order Zernike terms may still be present, making
corrections in later optical finishing steps more complex or even impossible.
Active homogenization typically yields a higher P-V variation. But the dominant contribution of this variation is the power term. Without the power term, the variation of the refractive index has a high rotational symmetry that can be further reduced during optical finishing steps
to yield higher-performance optics
(Figure 4).

Figure 4. An example of two fused silica blanks with comparable optical homogeneity in their peak-to-valley (P-V) values, but with very
different residual fluctuations in their refractive index. The data-free center originates in the
‘oil-on’ measurement. Courtesy of Heraeus.
Bubbles and inclusions
Other properties that can affect the performance of fused silica include bubbles and inclusions, which scatter light and are generally undesirable. The production process is typically designed for a smooth deposition to produce a homogeneous material without any bubbles. Bubbles do occasionally form, however, due to various reasons, including a disturbance during deposition, microscopic particles of dust, or insulation and impurities in the process gases.
Only optical systems designers can judge the relative benefits that higher performance grades can offer for their product and whether customers are willing
to pay for superior performance.
Once bubbles have formed, they can be removed only by cutting out a complete section of the material.
Any hot rework (that is, reworking only a portion of the batch) will yield a localized, strongly variant optical homogeneity that is distinguished from the remaining piece.
It is therefore general practice to specify the maximum permissible number and size of bubbles per optical blank — for example, following international standards such as ISO 10110. In this standard, the area obscured by bubbles and the
corresponding size of the bubbles are defined by size classes.
Historically, fused silica producers have not applied this definition to complete optical blanks but rather to any
100 cm3 of the material. This could obviously present problems for optics larger than 100 cm3, such as a cube of fused silica measuring 4.6 × 4.6 × 4.6 cm. In practical terms, however, the bubble content of fused silica is generally more negligible than the 100 cm3 reference
suggests. Nevertheless, it is worth checking these specifications.
Another consideration is striae, which are strong local changes in the refractive index. These variations are also a result of the production process of fused silica or subsequent process steps.
As mentioned earlier, the refractive index can be influenced by impurities or the volume of dopants, such as OH or H2 content. To generate a local change
in the refractive index, the concentration of these constituents must vary strongly along a very short lateral distance. Because manufacturers of fused silica aim to produce products that are as homogeneous as possible, striae typically occur during production of more extreme glasses (for example, water-free fused silica), or when geometries are manufactured outside the typical production portfolio.
Many variables during the production of fused silica can
influence the material’s properties. This explains why there are many different grades of fused silica.
But the relevance of this variety depends on the individual application. For most optical systems, basic UV or IR fused silica grades will work well, though higher performance is possible by selecting a more specific grade. Only optical systems designers can judge the relative benefits that higher performance grades can offer for their product and whether customers are willing to pay for superior performance.
Meet the author
Bernhard Franz, Ph.D., is product manager at Heraeus Conamic. He holds a doctorate in physical chemistry and has been working with fused silica for 14 years.