A co-registered photoacoustic/ultrasound imaging technique is emerging as a diagnostic tool, offering new hope for earlier ovarian cancer detection and diagnosis.
QUING ZHU, WASHINGTON UNIVERSITY IN ST. LOUIS
Since Bell Labs first described the photoacoustic effect — the conversion of light to sound — in the late 1800s1, researchers have explored the principle for medical imaging purposes2,3. Over the past decade, advancements in lasers, ultrasound transducers, and tomographic reconstruction techniques have prompted immense growth in photoacoustic imaging, a hybrid imaging technology that uses a short-pulse laser to excite tissue.
The resulting acoustic (or photoacoustic) waves are generated from thermoelastic expansion due to transient rising temperature. They are then measured by ultrasound transducers. The acquired photoacoustic waves are used to image, at ultrasound resolution, the optical absorption distribution, which in turn reveals optical contrast. Photoacoustic imaging has provided unprecedented spatial resolution and functional information at depths ranging from several millimeters to several centimeters.
Technology such as photoacoustic/ultrasound imaging that could reliably screen and diagnose ovarian cancer earlier may reduce the currently high mortality rate.
Optical contrast is directly related to microvessel networks and thus to tumor angiogenesis, a key process for tumor growth and metastasis. If two or more optical wavelengths are employed, photoacoustic waves can be used to reconstruct the distribution of blood oxygen saturation (sO2), which is an important indicator of tumor metabolism and therapeutic response.
To date, oncologic targets of photoacoustic imaging include several cancers: breast, prostate, skin, thyroid, and ovarian. The technology’s penetration depth is tunable with ultrasound frequency. In the diagnostic ultrasound frequency range of 3 to 10 MHz and with a spatial resolution of 150 to 500 μm, the penetration depth in tissue can reach 5 cm or more in the NIR spectrum, depending on the laser power, the ultrasound system’s sensitivity, and background tissue optical absorption and scattering. This penetration depth is adequate for transvaginal imaging of most ovarian masses.
Photoacoustic/ultrasound
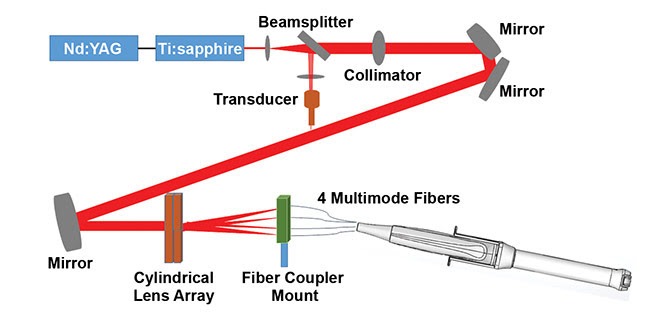
Figure 1. The light delivery path of the co-registered photoacoustic/ultrasound system for ovarian cancer imaging, developed by Washington University in St. Louis. Courtesy of Washington University in St. Louis.
Researchers at Washington University in St. Louis (WashU) developed a co-registered photoacoustic/ultrasound technique for noninvasive transvaginal examination of the ovaries4,5. A compact light delivery path (Figure 1) was designed. The system comprises a beamsplitter and collimator, mirrors, a lens array, four 1-mm-core multimode optical fibers sandwiched between an ultrasound transducer sheath, and a transducer to illuminate the ovaries via the vagina (Figure 2).
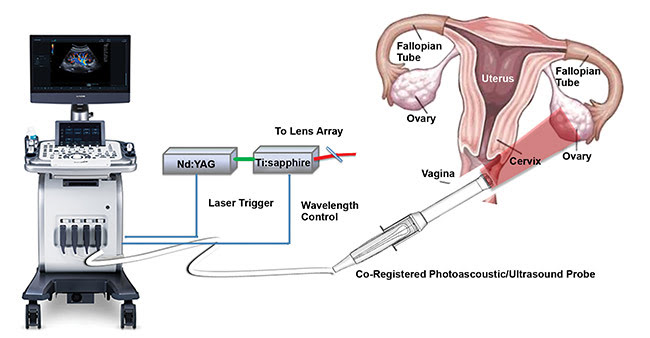
Figure 2. The co-registered photoacoustic/ultrasound system, developed by Washington University in St. Louis, and a diagram of the female reproductive system. Courtesy of Quing Zhu/Shutterstock.
Specifically, the lens array, which consists of four cylinder lenses, splits the incoming incident light into four beams, each of which is directed to couple into a 1-mm-core optical fiber. The illumination fibers are then affixed to the ultrasound probe, while the sheath encases both the transducer and the source fibers. The inner surface of the sheath and the outer surface of the ultrasound transducer are coated with an aluminum material having a reflection coefficient of 85 percent for high-intensity light output.
If two or more optical wavelengths are employed, photoacoustic waves can be used to reconstruct the distribution of blood oxygen saturation, an important indicator of tumor metabolism and therapeutic response.
Figure 2 shows the co-registered photoacoustic/ultrasound system used for our pilot patient study6. The system consists of three main parts: a fully programmable clinical ultrasound system (EC-12R, Alpinion Medical Systems Ltd. in South Korea); the customized optical fiber-based light delivery system coupled with the transvaginal ultrasound probe; and an Nd:YAG laser (Symphotics TII Corp.,
LS-2122, Camarillo, Calif.) pumping a pulsed, tunable (690 to 900 nm) Ti:sapphire laser (Symphotics TII,
LS-2134).
A time-division multiplexing approach is used during co-registered mode, wherein each photoacoustic imaging frame is synchronized with one laser pulse, and each ultrasound frame is recorded in the period between two consecutive laser pulses. Four wavelengths (730, 780, 800, and 830 nm) were used for patient study. These wavelengths were selected based on the absorption properties of oxygenated and deoxygenated hemoglobin, which are the main chromophores related to tumor angiogenesis and hypoxia.
Ovarian cancer detection
Ovarian cancer remains the deadliest of all gynecological malignancies. According to the American Cancer Society, just over 22,000 women were diagnosed with ovarian cancer in the U.S. in 2017, and about 14,000 deaths were reported. However, when ovarian cancer is detected at an early, localized stage (stages 1 or 2), surgery and chemotherapy cure 70 to
90 percent of patients, compared with
20 percent or fewer when it is diagnosed at later stages (stages 3 or 4)6.
Clearly, early detection is critical. Yet because of the lack of effective screening tools, only 20 to 25 percent of ovarian cancers are diagnosed early. Many women at high risk and/or with a screening abnormality will undergo prophylactic bilateral salpingo-oophorectomy (ovary removal), but this procedure is not appropriate for women with normal risk factors. (About 75 to 85 percent of women who develop ovarian cancer have normal risk factors.) Prophylactic salpingo-oophorectomy causes early menopause (if the patient has not already entered menopause), which carries risks, including accelerated bone loss and increased risk of heart disease. Premenopausal women up to a certain age who undergo the procedure and don’t use hormone therapy also have a higher rate of other diseases and premature death.
Current screening methods diagnose most women with ovarian cancer at stages 3 or 4, with widespread intra-abdominal disease; unfortunately, the majority of them will die of their disease.
Technology such as photoacoustic/ultrasound imaging that could reliably screen and diagnose ovarian cancer earlier may reduce this high mortality rate. Reducing the performance of unnecessary exploratory surgeries could also have a large impact. With an improved imaging method, noncancerous ovarian masses could be more easily identified. Thus, there is an urgent need to develop better and more sensitive tools to effectively evaluate the ovary.
Pilot study results
The first pilot patient study was reported in Radiology6. The group consisted of 16 patients enrolled at the Alvin J. Siteman Cancer Center at WashU. These patients were clinically at risk for ovarian cancer or had an ovarian or pelvic mass suggestive of malignancy. Prior to being imaged with the photoacoustic/ultrasound system, all patients were imaged by a radiologist using a GE commercial transvaginal ultrasound system — LOGIQ S8.
After the suspicious ovarian or pelvic masses were examined with the GE instrument, the commercial probe was withdrawn and the customized Alpinion photoacoustic/ultrasound system was inserted transvaginally to image them. Because the radiologists were familiar with the GE transvaginal imaging system, this two-step procedure was intended to ensure a smooth transition from the GE system to the Alpinion system. For each imaging location, five to 10 photoacoustic/ultrasound image frames were recorded for all four wavelengths. The overall acquisition time for all photoacoustic/ultrasound frames for four wavelengths at each location was about 12 to 14 seconds.
Two biomarkers were used to characterize the ovaries: relative total hemoglobin concentration (rHbT), which is directly related to tumor angiogenesis, and mean oxygen saturation (sO2).
In this pilot study, the researchers found the rHbT was 1.9× higher for invasive epithelial cancerous ovaries, which make up 90 percent of ovarian cancers, than for normal and benign ovaries. The mean oxygen saturation of invasive epithelial cancers was 9.1 percent lower than normal and benign ovaries. All nine invasive epithelial cancerous ovaries, including two stage 1 cancers and one stage
2, showed extensive rHbT distribution and lower sO2.

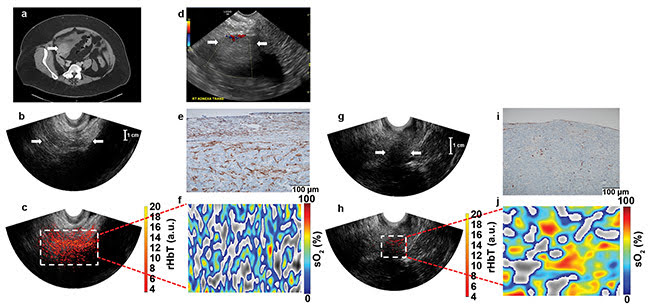
Figure 3. Contrast-enhanced CT chest/abdomen/pelvis examination of a 63-year-old postmenopausal patient with a solid right adnexal mass measuring up
to 4.5 cm, ascites, and a thickened endometrium (not shown). Pathologic findings on a 5-cm ovary with well-differentiated stage 2 endometrioid adenocarcinoma and an incidental 2.2-cm benign steroid cell tumor (a). Ultrasound Doppler of the right adnexa from GE LOGIQ S8 shows a hypoechoic soft tissue mass with minimal peripheral flow on color Doppler images (b). Ultrasound image of the right adnexa obtained from the Alpinion system (c), and the co-registered ultrasound and photoacoustic imaging rHbT map shown in color (d), with extensive diffused vascular distribution covering a large area of the region of interest (ROI) in the depth range of 1 to 4 cm. The rHbT measured in the ROI was 14.58 (a.u.). CD31 immunostain in the suture area, showing numerous and extensive micro-vessels (e). sO2 map of the ROI marked by the white rectangular box in d with mean sO2 of 43.2 percent (f). Ultrasound image of the left ovary of the same patient (g). Co-registered ultrasound and photoacoustic imaging rHbT map showing scattered photoacoustic imaging signals (h); the rHbT is much lower and
measured 7.73 (a.u.). CD31 immunostaining of surgical sample (i). sO2 map of the ROI identified by co-registered ultrasound with measured mean sO2 of
58.2 percent (j). Pathology found a normal ovary with no significant histopathologic abnormalities (see reference 6).
Courtesy of Washington University in St. Louis.
Figure 3 shows images from a 63-year-old postmenopausal patient with stage 2 endometrioid adenocarcinoma in the right ovary, and a normal left ovary. Figure 4 shows images from a 50-year-old premenopausal woman with bilateral well-differentiated stage 1 endometrioid adenocarcinomas.
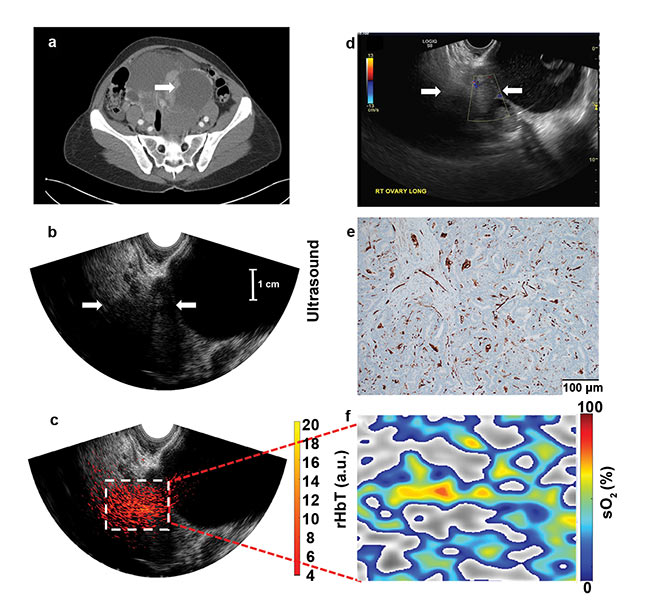
Figure 4. Images from a 50-year-old premenopausal woman with bilateral multicystic adnexal masses with septations and mural nodularity, revealed by contrast-enhanced CT; the arrow points to a 3.6-cm right adnexal mural nodule as a solid mass (a). An ultrasound Doppler image obtained by the GE LOGIQ S8 shows minimal blood flow in the solid area (b). Ultrasound image of the right adnexa (c). The co-registered ultrasound and photoacoustic imaging rHbT map shown in color, with extensive diffused vascular distribution inside the ROI in the depth range of 1.5 to 4.5 cm, next to large cystic areas identified by ultrasound (d). A CD31 immunostain of the sutured area (e). The sO2 map of the ROI, marked by the white rectangular box in d; pathology showed well-differentiated stage 1 endometrioid adenocarcinomas of both the right and left ovaries, which measured 8.3 cm and 20 cm, respectively; the rHbT measured in the ROI was 12.18 (a.u.), and the mean sO2 was 50.1 percent (see reference 6). Courtesy of Washington University in St. Louis.
The initial results with the dual imaging method have been encouraging. Next, WashU researchers will optimize the system and user interface to provide real-time photoacoustic/ultrasound imaging displays that will guide radiologists in diagnosing ovarian masses. They will then conduct a large-scale clinical study to validate the pilot data.
The technique will be endorsed for its potential role in optimizing the clinical management of low-suspicion, benign ovarian tissue abnormalities by reducing surgery recommendations without compromising cancer detection, thereby lowering morbidity and health care costs.
The potential of early detection and diagnosis of ovarian cancer will also be explored using the photoacoustic/ultrasound technique to follow a group of high-risk patients before their bilateral salpingo-oophorectomy. Monitoring this group of patients may lead to an initiation of an effective screening study for early ovarian cancer detection and diagnosis, with consequently much improved overall patient survival rates.
Meet the author
Quing Zhu is a professor in the Department of Biomedical Engineering at Washington University in St. Louis. She is a fellow of The Optical Society (OSA) and of SPIE, topical editor of Optic Letters (a publication of OSA), and an editorial board member of Photoacoustic and Biomedical Optics (an SPIE publication). Zhu’s research focuses on ultrasound-guided NIR imaging for breast cancer diagnosis and cancer treatment assessment and prediction; co-registered photoacoustic/ultrasound imaging for ovarian cancer detection and diagnosis; colorectal cancer treatment prediction; optical coherence tomography; and structured light imaging for cancer detection and diagnosis; email: [email protected].
References
1. A.G. Bell (1881). Production of sound by radiant energy. Science, Vol. 2, Issue 49, pp. 242-253.
2. L.V. Wang et al. (2012). Photoacoustic tomography: in vivo imaging from organelles to organs. Science, Vol. 335, Issue 6075, pp. 1458-1462.
3. A.A. Oraevsky et al. (2003). Optoacoustic tomography. In Biomedical Photonics Handbook. T. Vo-Dinh, ed. Boca Raton, Fla: CRC.
4. H.S. Salehi et al. (2014). Design of miniaturized illumination for transvaginal co-registered photoacoustic/ultrasound imaging. Biomed Opt Express, Vol. 5, Issue 9, pp. 3074-3079.
5. S. Nandy et al. (2018). Evaluation of ovarian cancer: initial application of coregistered photoacoustic tomography and US. Radiology, Vol. 289, Issue 3, pp. 740-747, https://doi.org/10.1148/radiol.2018180666.
6. National Cancer Institute. Surveillance, Epidemiology, and End Results Program, Cancer Stat Facts: Ovarian Cancer, www.seer.cancer.gov/statfacts/html/ovary.html.