The evolution of lasers has accelerated the use of photoacoustic spectroscopic applications in laboratory and medical settings.
FAROOQ AHMED, CONTRIBUTING EDITOR
Shortly before Alexander Graham Bell’s death in 1922, the Scottish American scientist and inventor said the telephone was not what he regarded as his greatest achievement. Rather, that distinction went to a now little-known device called the photophone1. In 1880, Bell and Charles Sumner Tainter used light to wirelessly transmit a voice message across a distance of 700 ft — roughly 100 years before the deployment of the world’s first cellular phone network.
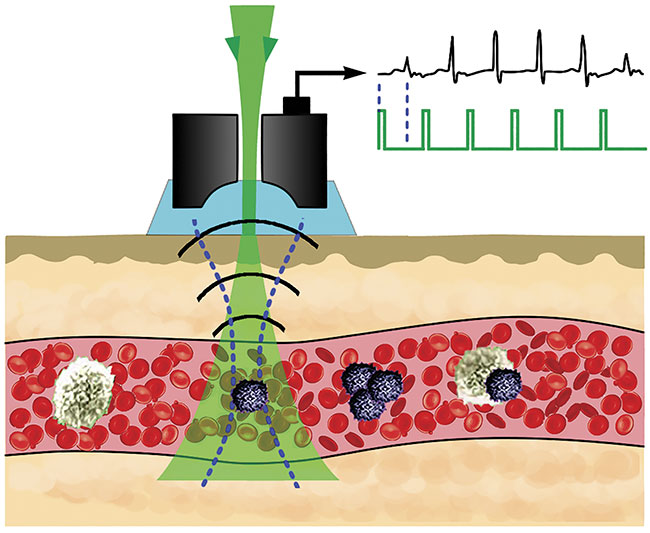
Vladimir Zharov of the University of Arkansas for Medical Sciences invented the Cytophone, which couples photoacoustics with flow cytometry to detect and kill skin cancer cells circulating in the bloodstream. Courtesy of Vladimir Zharov.
The technology didn’t gain traction on the market, however, and the telephone became an integral part of life in the 20th century. But the principle on which the photophone was based — using light to create sound, called photo- or optoacoustics — has in the past two decades given rise to a new generation of medical devices that promise noninvasive, label-free early detection of disorders and diseases including anemias, cancers, and atherosclerosis.
Laser power boosts usage
What changed was the development of the laser, according to Vladimir Zharov, the director of the Arkansas Nanomedicine Center at the University of Arkansas for Medical Sciences in Little Rock, Ark. Zharov has been using tunable lasers for photoacoustic spectroscopy since the 1970s. At that time, he was a researcher and later chairman of the biomedical engineering department at the Bauman Moscow State Technical University in the then-USSR. “The advantages come from power, which increases photoacoustic sensitivity thousands of times, regardless of application,” Zharov said.
The laser photoacoustics of the 1970s focused on industrial and environmental analysis, particularly using photoacoustic spectroscopy to analyze atmospheric pollution. This use persists: Earlier this year, several companies, including the German semiconductor manufacturer Infineon Technologies AG and Gasera Ltd. of Finland, announced the development of instruments that use photoacoustic spectroscopy to detect concentrations of carbon dioxide and volatile organic compounds in the air. Forty years ago, motivated by air pollution monitoring in the USSR, Zharov used lasers and photoacoustic detectors combined with chromatography to identify small molecules in the atmosphere and in exhaled air.
Photoacoustics in biomedicine
Photoacoustics provides multiple benefits that can be applied in biomedical applications. Laser light, for example, can noninvasively penetrate through the skin with depth and accuracy to image blood vessels, tissues, and organs. And unlike optical detection, acoustic detection functions well in biological tissues with high spatial resolution without fluorescent or other labeling methods.
“The key element behind photoacoustic and related photothermal techniques is energy conversion,” Andreas Mandelis said.
Mandelis is the director of the Center for Advanced Diffusion-Wave and Photoacoustic Technologies at the University of Toronto. “In many cases, you cannot simply use light to image, because of the effects of scattering and background interference,” he said. “However, if you use light as an input while detecting sound waves or thermal effects after light-to-ultrasound or light-to-heat energy conversion, the background is much lower, and you can get a huge dynamic range of signals.”
According to Mandelis, these techniques allow for increased resolution that can uncover subtle biological changes such as the buildup of arterial cholesterol that may otherwise go unnoticed.
The University of Arkansas’ Zharov agreed: “Soon after our experiments in environmental pollutants, we recognized the potential for this technology in the detection of diseases.” By the 1990s, buoyed by the development of tabletop-size lasers, Zharov said several research groups invented new schematics that led to an exponential growth in medical applications.
Mandelis made a similar observation: “Now, biomedical photoacoustics has virtually taken over the entire field.”
Enhanced imaging potential
Lihong Wang, now a professor of medical and electrical engineering at the California Institute of Technology (Caltech), led one of the groups that pioneered major developments in the field. In 2003, his lab demonstrated photoacoustic tomography could noninvasively image brain activity through the intact skin and skulls of live rats2.
“Our results were very much like functional MRI. That was a kind of paradigm shift in terms of using photoacoustics for biomedicine,” Wang said. “The field has been doubling every four years since that paper appeared.” Published in Nature Biotechnology, it remains the most cited article in the field.
Mandelis said Wang’s group “has been like a Swiss Army knife — developing many different tools and approaches to benefit the photoacoustic community.”
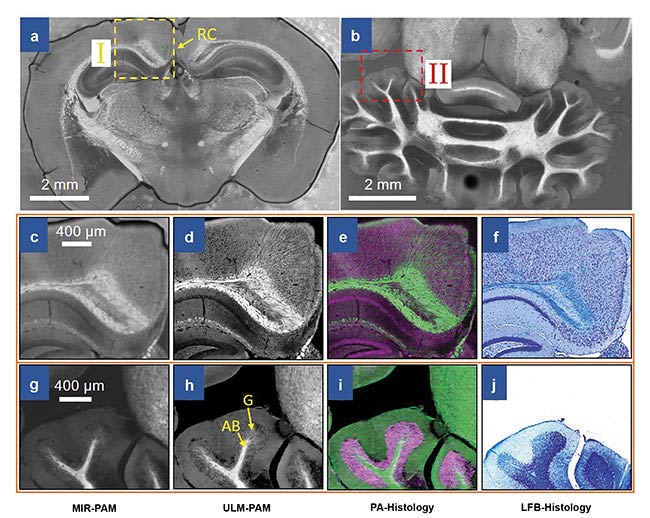
Imaging of mouse brain slices using mid-infrared photoacoustic microscopy (MIR-PAM). MIR-PAM images of myelin in 300-µm-thick slices of the cerebrum (a) and the cerebellum (b). Close-up MIR-PAM image of myelin (c). Ultraviolet-localized MIR photoacoustic microscopy (ULM-PAM) image of myelin (d). Photoacoustic (PA)-histologic image (e). Luxol fast blue (LFB)-stained histologic image (f) of the same area (I) shown in the cerebrum image (a). Close-up MIR-PAM image of myelin (g). ULM-PAM image of myelin (h). PA-histologic image (i). LFB-stained histologic image (j) of the same area (II) shown in the cerebellum image (b). In the PA-histologic images, green represents myelin and violet represents nucleic acids. In the LFB-stained histologic images, blue represents myelin and deep blue represents nucleic acids. RC: retrosplenial cortex; AB: arbor vitae region; G: granular region. Courtesy of Lihong Wang.
This year alone, the Caltech scientist’s lab has published research that uses photoacoustic microscopy to image metabolic heterogeneity within individual tumor cells3; it has used photoacoustic computed
tomography to look at blood flow in vessels in human extremities4; and it has used mid-infrared wavelengths to image biological samples with UV-localized photoacoustic microscopy5, among other research.
However, Wang started out using microwaves to generate acoustic waves, not lasers. “Our motivation was to visualize human tissues, but we couldn’t get the contrast we wanted because microwave wavelength is too long,” he said. After switching to a pulsed laser setup, Wang was able to perform functional imaging with both spatial resolution and high contrast, which led to the publication
of the seminal Nature Biotechnology paper.
Another benefit of photoacoustics in biomedical imaging is its ability to provide consistent light-absorption contrast on macro- and microscales. As a follow-up to the 2003 paper, in 2006 Wang’s group developed and wrote about a counterpart functional microscopy technique with which they demonstrated in vivo imaging of angiogenesis, melanoma, and hemoglobin oxygen saturation in small animals6. It remains the second most-cited paper in the field.
Wang added that detection technology,
specifically multichannel ultrasound transducers, has also contributed to the
growth of the field. “In our 2003 paper, we used a single-element ultrasonic transducer that took 20 minutes to acquire a data set,” he said. “Now, we fire a single laser pulse and get an equivalent data set in 50 microseconds in small animals.”
Enhanced detection has clinical relevance as well. Wang and others, such as fellow biomedical photoacoustic pioneer Alexander Oraevsky, the president and chief technology officer of Houston-based TomoWave Laboratories Inc., have been imaging the human breast for cancer studies. Rapid ultrasound has allowed for visualization to take place within a single breath hold, which greatly reduces artifacts. “The whole field has been working really hard in that direction,” said Wang. Recent ultrasound detectors have more than 500 channels operating in parallel. “Manufacturing this device is not something an academic research lab would do,” Wang said.
Detecting and killing cancer
In June, Zharov and others detailed the use of a device that couples photoacoustics with flow cytometry to noninvasively detect skin cancer cells circulating in vivo in the bloodstream in humans7. The Cytophone, as it is called, could be used for the early detection of metastatic skin cancer. And because the instrument does not rely on a blood draw, it can scan large blood volumes. “We can detect one bad cell per liter of blood,” said Zharov.
In the U.S. alone, one patient dies from melanoma every hour, according to Zharov’s paper.
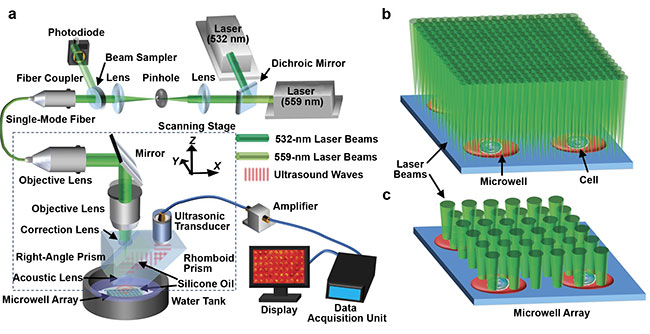
Lihong Wang of the California Institute of Technology developed a photoacoustic microscopy method to image metabolic heterogeneity within individual
tumor cells. System schematic of single-cell metabolic photoacoustic microscopy (SCM-PAM) (a). High-resolution mode of SCM-PAM, with optical diffraction
limited lateral resolution (b). High-throughput mode of SCM-PAM, with single-cell metabolism measurement throughput of ~3000 cells over 15 min (c).
Courtesy of Lihong Wang.
The Cytophone deploys a short, near-infrared laser pulse with a linear beam geometry to penetrate through intact skin and into blood vessels. Circulating melanoma cells, which contain nanoparticle-size melanin aggregates, preferentially absorb the light over red blood cells and other background. The absorbed light transforms first to heat, and this thermal expansion in circulating melanoma cells produces acoustic waves, which the Cytophone captures with ultrasound transducers on a patient’s skin.
The Cytophone is expanding into wider clinical trials in the U.S. It may also have theranostic applications. When using the device, Zharov’s group noticed a decrease in signals from circulating melanoma cells. Further investigation revealed that laser-induced nanobubbles that form around overheated melanin granules can amplify the sound wave and disrupt circulating melanoma cell structures, which leads to cell death.
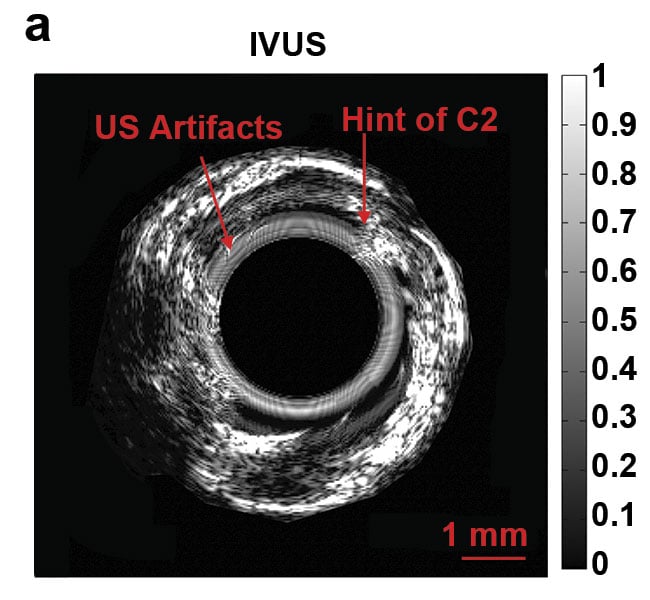
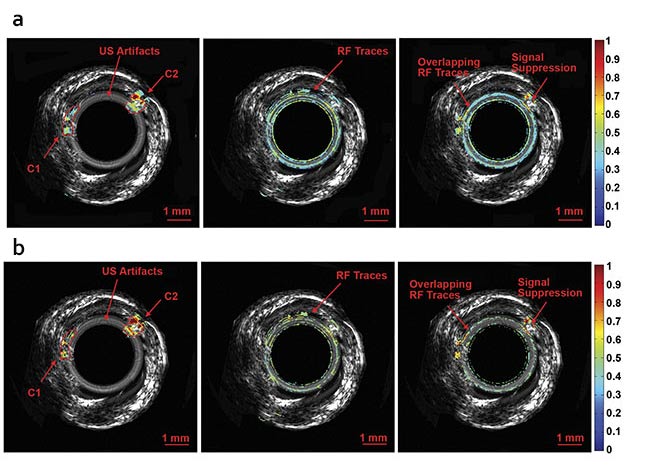
Normalized intravascular ultrasound (IVUS) image of the swine atherosclerotic artery phantom (a).
Normalized amplitude images (b) and normalized phase images of IV-differential photoacoustic radar (DPAR), 980-nm IV-PAR, and 1210-nm IV-PAR modes (c). The same 14-MHz endoscopic transducer was shared by IVUS and IV-(D)PAR systems for co-registration. C1: artificial cholesterol 1; C2: artificial
cholesterol 2; RF: radio frequency. Courtesy of Andreas Mandelis.
Thus, the Cytophone could potentially be used both to detect and to destroy malignant skin cancer cells in the bloodstream. “And we can kill only these specific targets without harming surrounding cells,” said. Zharov. Trials are underway to use the device for this treatment method, and Zharov anticipates training the instrument on other illnesses that manifest in the blood, including malaria, sickle cell and other anemias, diabetes, and stroke.
Zharov also has high hopes for miniaturization of the device. He’s working to develop a battery-based, wearable version of the Cytophone that may emerge as a cancer-killing smartwatch or allow for the noninvasive, continuous monitoring of blood parameters, such as glucose.
Power vs. frequency
Although many laser photoacoustic methods, such as Zharov’s, use pulsed lasers, the University of Toronto’s Mandelis developed a technique to image cholesterol buildup in arterial plaques with a continuous wave laser8. “Pulsed lasers are like optical hammers,” Mandelis said, “and they deliver strong signals. With a continuous-wave laser, you trade power for the ability to modulate frequencies and optimize signals using waveform engineering.”
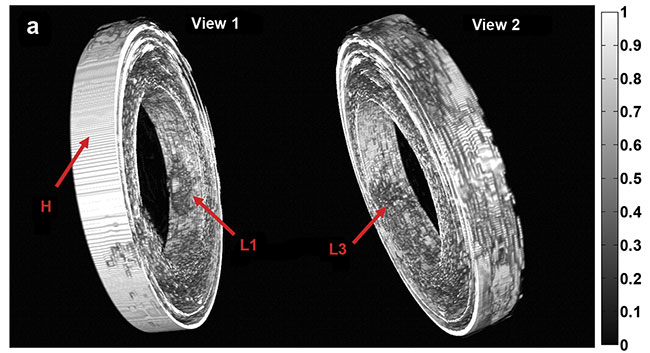
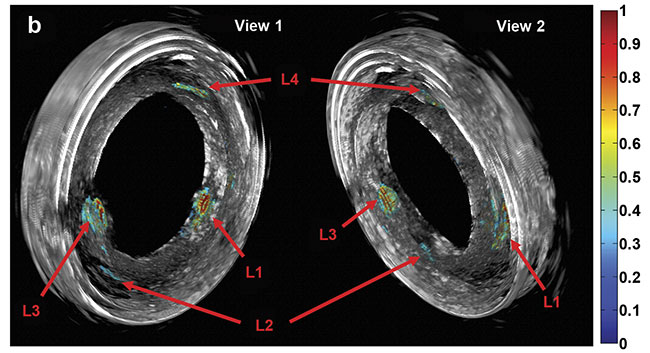
Purely 3D IVUS views (a) and co-registered 3D IV-DPAR/IVUS views of the swine artery phantom (b).
In the co-registered images, location and detailed depth distribution of artificial plaques were
color-coded on the gray-scaled IVUS background. L1-4: artificial plaques; H: plastic holder.
Courtesy of Andreas Mandelis.
Published in June, the research details how Mandelis’ team deployed light of multiple, near-infrared wavelengths into porcine arteries with an endoscopic probe and then analyzed the acoustic signals with radar methods. “This method produces molecular and mechanical contrast. Ultrasound provides that structure, and photoacoustics adds specificity,”
he said.
The early detection of atherosclerosis has the potential to greatly reduce the risk of heart attack and stroke. And according to Mandelis, differential photoacoustic radar, a modality that automatically subtracts signals at two light wavelengths, may also be able to image the colon and the gastrointestinal tract in a minimally invasive manner and track disease progression in other organs and in tumors at very early stages in their formation. Like Zharov, Mandelis is working to further miniaturize the endoscopic device and move into clinical trials.
Acknowledgments
The author would like to thank Vladimir
Zharov, the University of Arkansas for
Medical Sciences; Andreas Mandelis, the University of Toronto; and Lihong Wang, the California Institute of Technology.
References
1. F. Mims (Feb. 10-26, 1982). The first century of lightwave communications. Fiber Optics Weekly Update, pp. 6-23.
2. X. Wang et al. (2003). Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging
of the brain. Nat Biotechnol, Vol. 21,
pp. 803-806.
3. P. Hai et al. (2019). High-throughput, label-free, single-cell photoacoustic microscopy of intratumoral metabolic heterogeneity.
Nat Biomed Eng, Vol. 3, pp. 381-391.
4. P. Wray (2019). Photoacoustic computed tomography of human extremities. J Biomed Opt, Vol. 24, Issue 2.
5. J. Shi et al. (2019). High-resolution, high-contrast mid-infrared imaging of fresh biological samples with ultraviolet-localized photoacoustic microscopy. Nat Photonics, Vol. 13, Issue 9.
6. H.F. Zhang et al. (2006). Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat Biotechnol, Vol. 24, Issue 7, p. 848.
7. E.I. Galanzha et al. (2019). In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci Transl Med, Vol. 11, Issue 496.
8. S.S.S. Choi et al. (2019). Frequency-domain differential photoacoustic radar: theory and validation for ultrasensitive atherosclerotic plaque imaging. J Biomed Opt, Vol. 24,
Issue 6, pp. 1-12.