A combination of algorithms is used to identify cancer.
Dr. Sanford H. Barsky, Ohio State University College of Medicine and BioImagene Inc.
Digital techniques have taken center stage in clinical medicine as well as in biomedical research. Digital telemedicine, radiology and cardiology have replaced traditional subjective diagnostic modalities in their respective specialties.
Pathology, the interpretation of disease patterns under the microscope, until recently had not begun to embrace this digital revolution. For more than 150 years, pathologists have diagnosed diseases based on interpretations of visual patterns produced by passing light through translucent tissue sections. The subjective nature of this work meant that interobserver variability (variance among pathologists’ interpretations of the same slide set), intraobserver variability (variance among the same pathologists’ interpretations of the same slide set over time) and fatigue variability often confounded accurate disease interpretation. Technological developments, however, have begun to catalyze progress and wider acceptance of digital pathology.
The first development was a high-throughput microscopic slide scanner that can convert traditional glass microscopic slides into digital images that can be stored, retrieved, shared through the Internet and, most importantly, algorithmically analyzed. The other development was the creation of algorithms that can identify and quantitate immunocytochemical staining patterns and specific histological features, such as nuclear size and mitosis.
The algorithms can be applied to digital images of both whole microscopic slides and tissue microarrays. Tissue microarrays are a form of high-throughput screening, whereby numerous tissue biopsies from hundreds of patients can be analyzed on a single microscopic slide (or on an image of the slide). High-throughput screening is being used heavily in biomedical research: Oligo-spotted and cDNA microarrays, proteomic blots and tissue microarrays are high-throughput methods that allow biomedical researchers to analyze the DNA, RNA and protein content of patient biospecimens (tissues and fluids).
Achieving personalized medicine (individualized patient treatment) within the next decade will require the discovery of markers that guide each person’s therapy. The ultimate proof that a biomarker discovered by genomic or proteomic approaches is really tied to a disease or other condition is an evaluation of its pattern of in situ tissue expression, which only tissue microarrays can provide. Digital pathology and imaging technology that can scan tissue microarrays into “virtual slides” with high resolution and that can analyze their images algorithmically will accelerate the discovery of predictive biomarkers.
In recent studies, we applied our epithelial- and specific-recognition algorithms to whole slides and tissue microarrays from human cases of breast, colon and lung cancer. We analyzed each case for two nuclear, cytoplasmic and membrane immunocytochemical markers that were either homogeneously or heterogeneously expressed and compared the algorithmic measurements with the subjective ones.1,2 We successfully created both epithelial-recognition algorithms based on selective imaging properties (Gaussian kernel and elongation ratio) and specific-recognition algorithms based on pixel colors (RGB) and gray-scale intensities that can analyze both immunocytochemical staining and specific histological features.
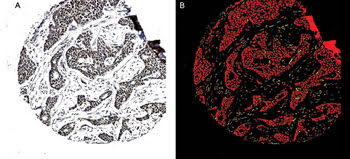
Figure 1. A breast cancer tissue sample (A) is subjected to image processing and the epithelial-recognition algorithms. The carcinoma cells are shown as red and the stromal cells as yellow (B). An applied filter could remove the noncarcinomatous areas, allowing application of the subsequent immunocytochemical specific-recognition algorithms to the carcinoma cells only.
Compared with traditional subjective pathological interpretation, the algorithms were better able to identify cancer cells in a background of stroma (Figure 1), to successfully compartmentalize the cancer cells into nucleus, cytoplasm and membrane, and to accurately and rapidly quantitate the degree of immunocytochemical staining (Figure 2). The illustrations depict one of the many immunocytochemical markers that were studied; in this case, estrogen receptor in human breast cancer.
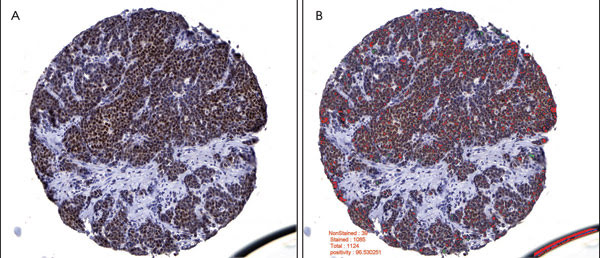
Figure 2. Breast carcinoma image analysis is depicted for an estrogen receptor, or nuclear biomarker. A tissue core showing positive estrogen receptor immunoreactivity (A) was preprocessed by mask removal and contrast enhancement, followed by epithelial area identification. Tumor nuclei were imaged in this epithelial area only. Based on whether they had more red than blue pixels, the estrogen receptor positive nuclei were labeled as red and the negative nuclei as green (B). The epithelial area was calculated at 80.376 percent, the estrogen receptor positivity at 96.5303 percent, and the median nuclear intensity of the positive nuclei at 94 (0, most intense; 255, least intense).
These algorithmic approaches establish a proof of principle that diverse tissue patterns of disease can be studied and analyzed by artificial intelligence.
Meet the author
Dr. Sanford H. Barsky is the Donald A. Senhauser endowed chair of pathology at Ohio State University College of Medicine in Columbus and serves as medical director of BioImagene Inc. in Cupertino, Calif.; e-mail: [email protected].
References
1. G.M. Sharangpani et al (June 2007). Semi-automated imaging system to quantitate estrogen and progesterone receptor immunoreactivity in human breast cancer. J MICROS, pp. 244-255.
2. A.S. Joshi et al (May 2007). Semi-automated imaging system to quantitate Her-2/neu membrane receptor immunoreactivity in human breast cancer. CYTOMETRY A, pp. 273-285.