
Atomic Clock Determines Absolute Timing of Photoelectric Effect
According to scientists at the University of Vienna (TU Wien), it is now possible to measure the photoelectric effect. The TU Wien team, together with research groups from Garching, Munich, and Berlin, determined the duration of the photoelectric effect using a tungsten surface.
When light falls on certain materials, electrons are released from their surface. In attoseconds, an electron from the material will absorb a photon, “jump” into another state, and leave the surface. This phenomenon, or photoelectric effect, is so fast that until now it has mostly been regarded as instantaneous.
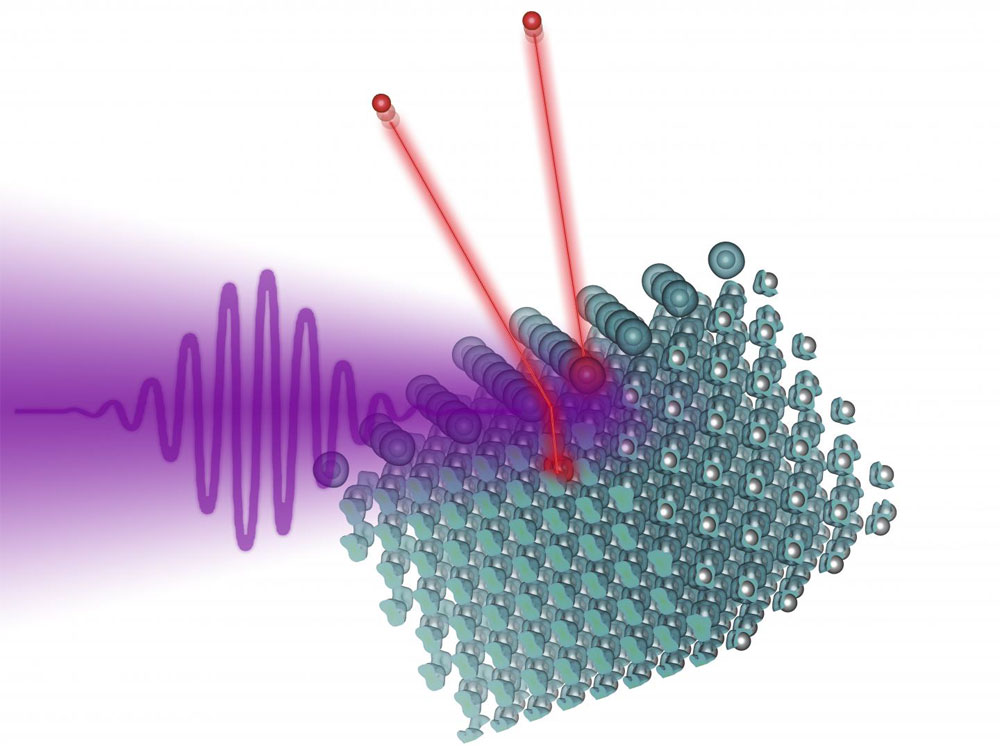
A laser pulse hits a tungsten surface on which iodine atoms have been deposited. Both the tungsten atoms and the iodine atoms lose electrons, which can then be measured. Courtesy of TU Wien.
While scientists have been able to determine the time interval between different quantum jumps and show that these different jumps take different amounts of time, until now it has only been possible to determine time differences, not the absolute duration of the process.
Using the atomic chronoscope method, which references all measured timings to the moment of light-pulse arrival, the researchers were able to determine the absolute timing of the photoelectric effect. Through experiments, computer simulations, and theoretical calculations, they discovered and defined an atomic clock that starts precisely at the beginning of the quantum jump.
To achieve an absolute, precisely calibrated reference scale, the researchers began by studying electrons removed from helium atoms by laser pulses. They used the helium atoms as a reference clock.
“The helium atom is very simple. In this case, we can accurately calculate the time evolution of the photoemission,” said professor Christoph Lemell.
In their second experiment, the researchers compared the photoemission of helium and iodine, using the comparison to calibrate an “iodine clock.” In the third and final experiment, the researchers used the iodine atoms to study the photoemission of electrons from a tungsten surface.
The researchers deposited the iodine atoms on a tungsten surface and irradiated the surface with ultrashort laser pulses. The ultrashort pulse was the starting signal for the photoelectric effect to begin. When the pulse hit the surface of the tungsten, electrons were released from their atoms and jumped to a different quantum state, a state in which they could reach the tungsten surface and leave the surface.
The iodine atoms, serving as a reference clock, were used to measure the photoemission from the tungsten surface.
“In tungsten, the duration of this process can be studied particularly well because the interface of the material can be defined very precisely there. The tungsten surface is an excellent finish line for electron-time measurement,” said professor Florian Libisch.
The team said that the duration of the photoemission process depends on the initial state of the electrons. It ranges from 100 attoseconds for electrons from the inner shells of the tungsten atoms to 45 attoseconds for conduction band electrons, which on average pass the finish line faster.
The researchers’ goals extend beyond purely measuring the duration of a quantum effect. The photoelectric effect plays an important role in many fields, for example in solar cells or in the conversion of data from a fiber optic cable into electrical signals.
"It is an exciting field of research that provides remarkable new insights — for example into surface physics, and into electron transport processes inside materials,” said professor Joachim Burgdörfer. “It gives us the opportunity to study important physical processes with an accuracy that would have been inconceivable a few years ago.”
The measurements were carried out at the Max Planck Institute for Quantum Optics. Scientists from the TU Wien were responsible for the theoretical work and computer simulations.
The research was published in Nature (doi:10.1038/s41586-018-0503-6).
Published: September 2018