The technology reveals changes in murine fetal brain vasculature caused by teratogens. These alterations can be precursors to later health problems.
Rajesh C. Miranda, Raksha Raghunathan and Kirill V. Larin, University of Houston
No amount of alcohol is considered safe during pregnancy, according to published research1. Despite this message being the subject of constant public service warnings, in a recent study, 20% to 30% of women in the U.S. reported drinking during pregnancy. Fetal alcohol spectrum disorders (FASDs) refer to the broad spectrum of behavioral and developmental deficits caused by prenatal exposure to alcohol. FASDs are common, with a global prevalence of 22.7 per 1000 births. Persons with these disorders can exhibit a range of deficits, from mild to severe. FASDs are highly underdiagnosed1, and they can be difficult for medical practitioners to differentiate from other developmental disorders.
Courtesy of iStock.com/ninjamonkeystudio.
Thanks to modern technology, angiographic optical coherence tomography (OCT) is helping to diagnose these disorders before they arise by extracting information about the developing fetus early. The application of this technology and the knowledge gained in early diagnosis could provide benefits to fetal and infant health by facilitating preventive measures that can mitigate damage.
OCT is an interferometric technique capable of label-free, noninvasive, and depth-resolved imaging of tissues with micrometer-scale spatial resolution. The technique is considered an optical analog of ultrasound, and it uses backscattered light from various layers of a sample to obtain depth-resolved information.
The technique can also help to identify complications associated with prenatal nicotine use. Nicotine exposure, like alcohol exposure, is known to pose several health risks to a developing fetus: Preterm birth, low birth weight, and intrauterine growth restriction — and, later, sudden infant death syndrome — are a few common effects. Nicotine is known to affect brain development, with human studies showing behavioral changes in babies exposed before birth, as well as cause a decrease in overall brain volume2.
Synthetic cannabinoids (SCBs) are a group of heterogeneous compounds that were developed to help with understanding the endogenous cannabinoid system and for use as potential therapeutics3. Their usage has increased for a number of reasons: They provide psychoactive effects similar to those of natural cannabinoids, they are available in ready-to-use formulations that are easily accessible over the internet and in specialty shops, and they are undetectable in routine drug screenings. SCBs have a 40- to 600-fold higher potency than natural cannabinoids and hence are more toxic. Recent studies have reported an increase in the use of natural cannabinoids and SCBs by women of reproductive age. However, little research has been done to assess the effects of prenatal exposure to SCBs.
Prenatal exposure to alcohol and drugs is a major public health concern because it is one of the main causes of congenital birth defects. The severity of the abnormality depends upon the amount of the substance used and the period of gestation during which the drug use happens. Due to unintended pregnancies and late recognition of pregnancies, some women continue to use these substances well into, and even past, the second trimester.
The mid-first through the second trimesters mark the peak period for fetal neurogenesis and angiogenesis. The microvasculature that penetrates the fetal brain during the second trimester of pregnancy is known to support the nutritional needs of the embryo4, provide endocrinal support5, and promote neural development6. Thus, it is important to study the effects of prenatal exposure to teratogens — factors or agents that may cause malformations in an embryo or fetus — when such exposure occurs during this trimester of pregnancy. Several studies have documented morphological and behavioral changes in babies after prenatal exposure to various drugs during this trimester. However, acute changes in vasculature have not been documented.
Rapid acquisition speeds
Since its introduction in 19917, OCT has been used in a variety of fields, including ophthalmology, oncology, dermatology, and cardiology.
Because it is a label-free and noninvasive technique with rapid acquisition speeds, high-resolution imaging capability, and the ability to provide live cross-sectional images, OCT has also been successfully used for small-animal embryonic imaging over the past two decades8.
Embryogenesis is a highly complex and dynamic process. To understand basic physiological processes that happen during embryogenesis and to evaluate possible defects, a method of visualization is crucial. Several imaging modalities have been used for embryonic imaging over the years. Histological sectioning has been the gold standard for phenotypic analysis. However, because this method is invasive and time-consuming, it is unsuitable for live imaging. Moreover, the process of preparing the embryo or fetus for study can alter the gross morphology.
Other noninvasive imaging modalities — such as ultrasound biomicroscopy (UBM), micro-magnetic resonance imaging (micro-MRI), and micro-computed tomography (micro-CT) — have been used for embryonic imaging. UBM has an imaging depth of a few centimeters, but its limited spatial resolution of 30 to 100 µm makes it unsuitable for imaging smaller structures and processes. Micro-MRI’s long acquisition times and its use of external contrast agents make it unsuitable for imaging small embryos. Although micro-CT offers a high spatial resolution of 2 to 50 μm, its use of ionizing radiation makes it unsuitable for
live small embryo imaging. For these reasons, OCT has gained popularity over all these other imaging modalities for small-animal embryonic imaging.
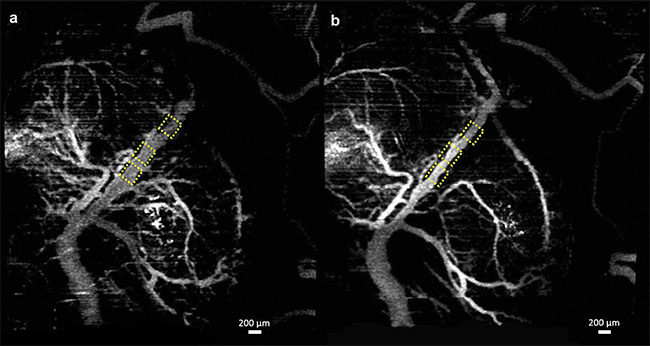
Figure 1. Vasculature changes in the embryonic brain before (a) and 45 min after (b) maternal exposure to ethanol. Adapted with permission from
Reference 10.
Over the years, several functional extensions of OCT have been developed to broaden the applications of the technology from mere structural imaging to functional imaging. Examples include Doppler OCT, which was developed to measure blood flow velocities, and optical coherence elastography, which was developed to measure changes in biomechanical properties of tissues. Another functional extension of OCT is angiographic OCT, which was introduced to image microvasculature9.
In recent studies, angiographic OCT was used to image murine fetal brain vasculature in utero10-12. A series of experiments documented changes in murine fetal brain vasculature minutes after maternal exposure to various drugs. Embryos of pregnant mice at embryonic day 14.5 were imaged. This period corresponds to the end of the first trimester and beginning of the second trimester in human development. Initial OCT imaging was performed, and the mother was administered the drug for the corresponding study. Subsequent measurements were taken of the embryo for a period of 45 min, at 5-min intervals.
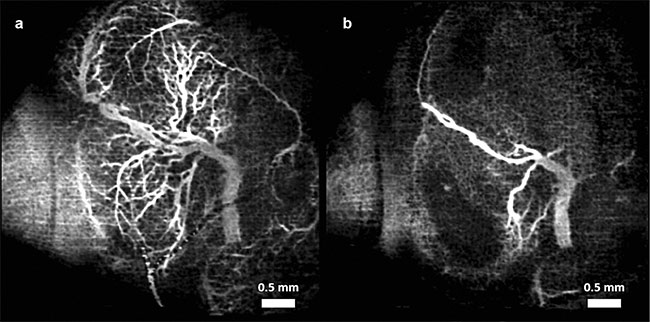
Figure 2. Vasculature changes in the embryonic brain before (a) and 45 min after (b) maternal exposure to nicotine. Adapted with permission from
Reference 11.
For the experiments that tested the effects of alcohol, 16.6% ethanol at a dose of 3 g/kg was used. This dose was based on gas chromatographic analysis of mice with blood alcohol concentrations that were similar to those observed in humans who engage in risky patterns of drinking, such as binge drinking, but do not yet have an alcohol use disorder or addiction13.
For the nicotine experiments, nicotine at a dose of 1 mg/kg was used. This dose in mice is known to cause intrauterine growth restriction. Both the alcohol and nicotine were administered via intragastric gavage. For the SCB studies, CP-55,940 at a dose of 2 mg/kg, suspended in a compound of dimethyl sulfoxide (DMSO) (Alkamuls El 620: lactated Ringer’s solution at a ratio of 1:1:18), was sprayed onto the liver of the mother. CP-55,940 was used because it is a well-known and well-characterized compound in SCB research.
Speckle variance OCT (svOCT) and correlation mapping optical coherence angiography (cmOCA) are two types of angiographic OCT techniques that were used in the studies. While svOCT calculates the variance between multiple images taken at the same spatial location, cmOCA calculates the correlation coefficient between the images to capture any dynamic scatterers (such as blood in this case) in the specific location. These procedures are followed at multiple consecutive locations to obtain a vasculature map. Once the 3D vasculature maps were obtained, the maximum intensity projections (MIPs) were calculated to obtain 2D images.
Figures 1-3 show the vasculature in the fetal brain before and after maternal exposure to ethanol, nicotine, and SCBs, respectively.
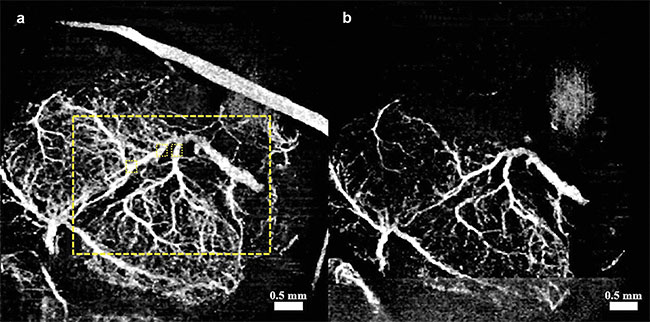
Figure 3. Vasculature changes in the embryonic brain before (a) and 45 min after (b) maternal exposure to synthetic cannabinoids (SCBs). Adapted
with permission from Reference 12.
In all three cases, when compared to placebo groups, a drastic reduction in vessel diameter was observed within 45 min of maternal exposure, demonstrating the possibility that all three teratogens act as a vasoconstrictor on the fetal brain. Such constriction could have a drastic effect on normal brain development.
These initial studies have opened up several new avenues to explore. For example, in the case of ethanol, a small concentration led to a drastic effect. This emphasized the need to study dose response curves at the same concentration of ethanol and then eventually at various concentrations.
Each of these studies reported on
observations made over a period of
45 mins following drug administration. It is important to know whether the demonstrated drug effects persist beyond a definite time point, and whether they are reversible. Other questions include: If the drug effects are reversible, does temporary vasoconstriction still cause permanent damage to the fetus? And can these effects be correlated to brain development, behavior, and cognitive and intellectual abilities in adulthood? Finally, the effects of co-abuse of these drugs need to be investigated because the use of two or more together may have synergistic effects and thus further reduce cerebral blood flow during the second trimester of development.
Meet the authors
Raksha Raghunathan is a postdoctoral fellow in the Biomedical Optics Laboratory at the University of Houston. Her research interests include understanding the process of embryogenesis and using noninvasive imaging methods to study the effects of various teratogens on embryogenesis; email: [email protected].
Rajesh C. Miranda is professor of neuroscience and experimental therapeutics at the Texas A&M University Health Science Center College of Medicine. His research focuses on developmental neurobiology, neural stem cells, epigenetics, and drug abuse, with a specific emphasis on fetal alcohol spectrum disorders; email: [email protected].
Kirill V. Larin is a Cullen College of Engineering Endowed Professor of Biomedical Engineering at the University of Houston. He received a doctorate in biomedical engineering from the University of Texas Medical Branch in Galveston. He has published more than 170 papers in the fields of biomedical optics and biophotonics. Larin is a fellow of SPIE, OSA, and AIMBE; email: [email protected].
References
1. J.F. Williams and A.V.C. Smith (2015).
Fetal alcohol spectrum disorders. Pediatrics, Vol. 136, Issue 5, pp. e1395-e1406.
2. A. Agrawal et al. (2010). The effects of maternal smoking during pregnancy on
offspring outcomes. Prev Med, Vol. 50,
Issue 1-2, pp. 13-18.
3. M.S. Castaneto et al. (2014). Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend, Vol. 144, pp. 12-41.
4. M.G. Norman and J.R. O’Kusky (1986).
The growth and development of micro-vasculature in human cerebral cortex.
J Neuropathol Exp Neurol, Vol. 45, Issue 3, pp. 222-232.
5. A.L. Fowden and A.J. Forhead (2009). Endocrine regulation of feto-placental growth. Horm Res, Vol. 72, pp. 257-265.
6. S.J. Tam and R.J. Watts (2010). Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci, Vol. 33, pp. 379-408.
7. D. Huang et al. (1991). Optical coherence tomography. Science, Vol. 254, Issue 5035, pp. 1178-1181.
8. R. Raghunathan et al. (2016). Optical coherence tomography for embryonic imaging:
a review. J Biomed Opt, Vol. 21, Issue 5,
p. 50902.
9. A.H. Kashani et al. (2017). Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res, Vol. 60, pp. 66-100.
10. R. Raghunathan et al. (2018). Evaluating the effects of maternal alcohol consumption on murine fetal brain vasculature using optical coherence tomography. J Biophotonics, Vol. 11, Issue 5, p. e201700238.
11. R. Raghunathan et al. (2020). Optical coherence tomography angiography to evaluate murine fetal brain vasculature changes caused by prenatal exposure to nicotine. Biomed Opt Exp, Vol. 11, Issue 7, p. e201900050.
12. R. Raghunathan et al. (2019). Assessing
the acute effects of prenatal synthetic
cannabinoid exposure on murine fetal
brain vasculature using optical coherence tomography. J Biophotonics, Vol. 12,
Issue 8, p. e201900050.
13. S. Bake et al. (2017). Fetal alcohol exposure alters blood flow and neurological responses to transient cerebral ischemia in adult mice. Alcohol Clin Exp Res, Vol. 41, Issue 1, pp. 117-127.