
Advances in Optical Coherence Tomography
Technological innovations with optical coherence tomography promise to serve a range of unmet market needs.
NISHANT MOHAN, WASATCH PHOTONICS
Optical coherence tomography (OCT) is a high-resolution, three-dimensional, noninvasive imaging technique. It is often called an optical ultrasound because it relies on time-of-flight information, similar to ultrasound imaging, to obtain subsurface information. The success of this technique has been fueled by a unique combination of technical and commercial factors, which include major investment in lasers, optical fibers, and sensors for telecommunications. That OCT is benefitting from these advances is proven in its applications across the health care industry.
With close to a billion-dollar market size, OCT is seeing increased acceptance in areas such as interventional cardiology, dermatology, and ophthalmology, and in nondestructive testing. In cardiology, the high-resolution and subsurface imaging capability of OCT is utilized in cases of acute cardiac events, such as stent placement, also known as percutaneous coronary intervention. A recently published study demonstrated that OCT is associated with fewer adverse events during the procedure, and more importantly, a statistically significant improvement in post-procedural mortality, after accounting for baseline differences1.
OCT is also providing solutions for important and growing needs in ophthalmic diagnostics. With an aging global population, there is a steady increase in the number of people with eye-related disorders and, in turn, a growth in demand for screening techniques. OCT, with its distinct capability to image retinal and corneal layers, has become the standard of care for conditions such as age-related macular degeneration, glaucoma, and diabetic retinopathy. The confluence of technical improvements and commercial need has resulted in continuous investment and growth in OCT, helping to extend its use in applications.

Figure 1. Growth in number of OCT procedures performed in the U.S. each year. See reference 4. Courtesy of Eric Swanson.
In ophthalmology, OCT imaging has extended application beyond standard diagnostic imaging. The high speed and high resolution of the technique can be used to guide several ophthalmic procedures, such as cataract surgery, vitroretinal surgical procedures, intraocular lens modification procedures, and laser-based vascular therapies (Figure 1). In addition, ophthalmic OCT scans are increasingly becoming valuable for assessment of neurological conditions such as multiple sclerosis and Alzheimer's disease2.
Compared to other 3D medical imaging technologies, OCT is inexpensive and easy to use. As of 2018, nearly one OCT eye scan is performed every second. The amount of data this generates is opening a host of interesting applications driven by artificial intelligence (AI). A recent study documenting a collaboration between Google's DeepMind and Moorfields Eye Hospital in London tracks their AI-based algorithms, which make automated referrals based on OCT images without any assistance from a physician3.
These phenomenal advances in OCT have key technological developments at their core. While invented in the early 1990s, OCT became practical for a variety of applications only after the advent of what is called Fourier domain OCT technology. This technology increased the imaging sensitivity and speed of OCT by orders of magnitude. The increase in speed allowed not only in vivo data collection without patient motion artifacts, but has resulted in large amounts of varied data.
Recent technological developments are powering the next leap in speed and sensitivity of OCT, particularly associated with the spectral domain submodality of the technique.
Spectral domain OCT
Spectral domain OCT (SD-OCT) was the first style of Fourier domain OCT to be commercialized. The technique utilizes a broadband light source to illuminate the sample. The resulting reflections are combined with a reference beam and the resultant interference is measured as a function of the wavelength, or frequency, with a spectrometer. Information from interference of several different frequency components is used to reconstruct the total time of flight and the depth profile of the sample. Such a technique promises very high-speed imaging by employing parallel detection of all different frequencies with a spectrometer. However, high-speed interferometric measurement puts specific constraints on the design of the spectrometer.
First, the measurement of the interference at different frequencies is extremely sensitive to any crosstalk between different frequency channels. Excessive crosstalk results in a loss of interference information, and the quality of the image is reduced. This is particularly true for high-frequency interference that corresponds to deeper parts of the sample. In effect, a lack of fidelity in spectrometer output results in lower image sensitivity deeper into the sample.
In addition, the spectrometer must output data at a much faster speed, in the order of 10 to 100 kHz, as compared to traditional models operating in orders of magnitude below this range. This requires sufficient signal to the sensor, along with sensor design that enables transfer of data at high speeds, without excessive noise. The spectrometer camera also should ensure minimal crosstalk between pixels to avoid degradation of the interference information, as discussed above. This high speed and low crosstalk require optimal design of both the spectrometer optics and the sensor.
Optical design
Spectrometer optics consists of dispersion and focusing elements that separate the light into different wavelengths, and then directs those wavelengths to individual pixels, respectively. To obtain high speed and sensitivity, the optics of the spectrometer needs to ensure high throughput to supply sufficient signal to the detector. And, to ensure deeper penetration, individual wavelengths should be focused to a tight spot without aberrations, avoid crosstalk, and provide improved sensitivity roll-off performance.
High throughput is obtained by use of high-dispersion volume phase holographic gratings. These elements separate different colors at sharp angles while significantly reducing the amount of light lost. The focusing optics convert angles to positions on the linear detector. The optical design needs to ensure that energy for a given wavelength is contained within one pixel. This requires optimizing OCT spectrometer optical design to unique constraints. Unlike with a standard imaging camera, the image quality in the central and peripheral fields is important.
Spectrometers use only line-scan cameras, and hence point spread function or mutual transfer function in the direction perpendicular to the sensor is not of significance. Figure 2 illustrates how a custom optical design helps focus the beam better onto a single pixel compared to an optical setup optimized with off-the-shelf lenses.
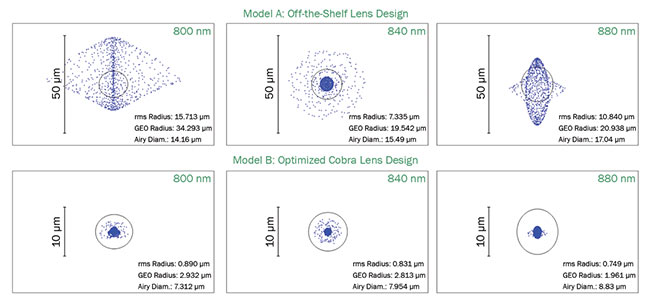
Figure 2. OCT spectrometer design optimizing spot size across different wavelength regions. A comparison between custom and off-the-shelf optics. Courtesy of Wasatch Photonics.
Sensor technology
Further improvements in the spectrometer come from choosing a light sensor ideally suited for OCT, where, rather than simply measuring reflected light intensity, interferometric signal is recorded. There are three main requirements for the OCT sensor: 1) fast readout speed, 2) large dynamic range, and 3) low crosstalk between adjacent pixels.
The high speed is obtained by choosing appropriate technology. The latest OCT sensors based on CMOS technology have dedicated analog-to-digital convertors per pixel that allow quick transfer of photoelectrons to digital counts. In addition, optimized designs allow the presence of high electric fields to enable fast discharge of accumulated electrons.
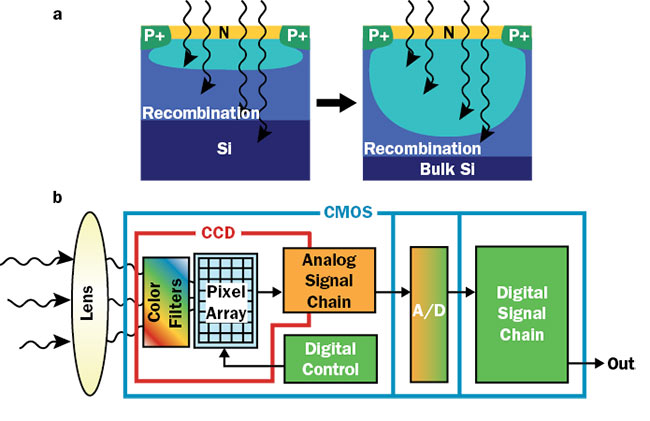
Figure 3. A large depletion zone allows absorption of longer wavelengths and reduced crosstalk between the neighboring pixels (a). CMOS sensor design incorporates dedicated analog-to-digital conversion allowing fast transfer of data (b). Courtesy of Teledyne e2v.
The high dynamic range in OCT imaging requires a sensor with low noise and large full-well capacity. This capacity of the sensor is directly related to the pixel capacitance for a given voltage. The pixel architecture is modified to add capacitance resulting in full-well capacity. The increased capacitance, in turn, has to be compensated for by increased electrical field of charge transfer to avoid leaving residual charge at high speeds.
The crosstalk in CMOS sensors is a result of electrons generated outside the depletion zone that are not guided by the applied electric field and can migrate toward surrounding pixels. A custom wafer substrate is used to increase the depletion zone and results in reduced crosstalk, particularly for the longer wavelength regions used in OCT (Figure 3).
High throughput and low-aberration optical design, combined with high speed, high dynamic range, and low crosstalk of the optical sensor, is powering the next generation of SD-OCT technology, which is already opening doors for applications.
Applications
Advancements that result in increased speed and imaging depth of SD-OCT are allowing researchers to explore an entirely new set of imaging applications - one relates to imaging human corneas and surrounding structures in an unprecedented manner. While OCT has been used in the past to image corneal layers at tissue level, recent developments allow imaging at a cellular level.
In vivo cellular imaging requires three-dimensional capture for useful interpretation of data. This, in turn, requires both high resolution and high speed to avoid the movement of the subject or patient during the imaging. Traditionally, SD-OCT has been used for high-resolution applications. However, limited imaging speed has previously prevented cellular-level in vivo imaging of corneas.
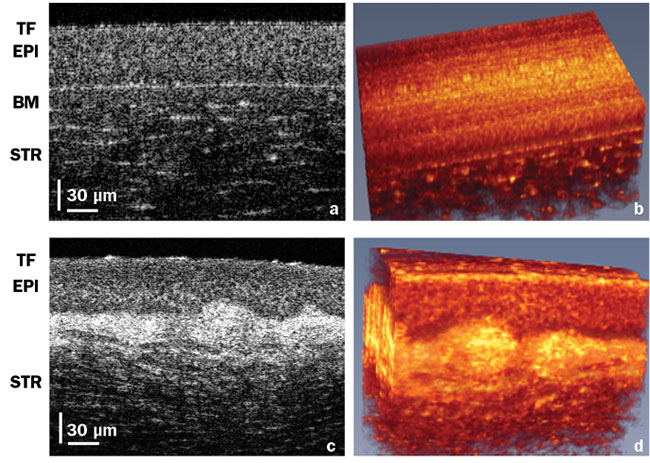
Figure 4. High-resolution corneal images. A cross section of a healthy cornea showing normal tear film (TF)(a). In vivo volume of the healthy cornea (b). Similar layers in a keratoconus patient showing irregular TF and loss of structure between epithelium and Bowman’s layer (c). In vivo volume of the keratoconus eye (d). EPI: epithelial layer; BM: Bowman’s layer; STR: stroma. Courtesy of Professor Kostadinka Buzheva, University of Waterloo.
At the University of Waterloo, Kostadinka Bizheva's group has used a high-speed Cobra-S spectrometer at a 250-kHz line rate to perform cellular-level imaging of corneas. The latest spectrometer technology allowed this high speed to be combined with an axial resolutionof 1.4 µm and transverse resolution of 2 µm.
Imaging corneas at a very high resolution is of great significance for several corneal diseases, including keratoconus, a condition that results in deformation and thinning of corneas. This deformation prevents the formation of a clear image on the retina and leads to poor vision for the patient.
The success of OCT has been fueled by a distinct combination of technical and commercial factors, which include major investment in lasers, optical fibers, and sensors for telecommunications.
Bizheva employed high-resolution and high-speed OCT to compare cellular-level differences between healthy and keratoconus eyes. The difference in OCT images for these two cases is illustrated in Figure 4.
Additionally, high-resolution, high-speed SD-OCT imaging can visualize both the structure and the dynamics of tear film on the very top of the cornea. The presence of healthy tear film (Figure 4a) is critical for good vision. Irregularities in the tear film (Figure 4c) are associated with dry eye disease, which affects over 3 million people in the U.S. alone.
The ability to image and quantify properties of corneal nerves, such as density, thickness, and tortuosity, can potentially open an entirely new way of diagnosing, managing, and studying systemic diseases such as diabetes.
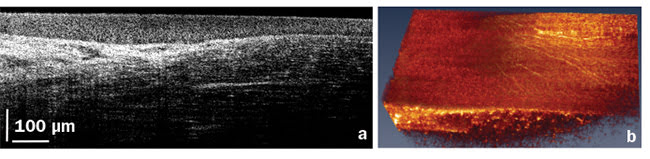
Figure 5. A high-resolution, high-speed corneal image acquired close to the limbal region. Cross section (a) and volumetric image (b) showing corneal nerves. Courtesy of Professor Kostadinka Buzheva, University of Waterloo.
OCT has proven to be an essential tool for ophthalmology, with increased acceptance in several other applications. Over the last two decades, technological advances have spurred the growth and acceptance of OCT. The latest developments in spectrometer technology have provided major leaps in the ability to address entirely new sets of unmet needs. This self-sustaining cycle of technological innovation and expansion of application domain continues to fuel the growth of OCT.
Meet the author
Nishant Mohan is vice president of the OCT division at Wasatch Photonics. He has actively participated in strategy, marketing, research, and development of OCT for more than a decade. Prior to joining Wasatch, Mohan was part of the R&D team of Bausch & Lomb Inc. in Rochester, N.Y., and a research fellow at Harvard Medical School.
References
1. D.A. Jones (2018). Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: Outcomes from the PanLondon PCI cohort. JACC Cardiovasc Interv, Vol. 11, Issue 14, pp. 1313-1321.
2. B.E. O’Bryhim (2018). Association of preclinical Alzheimer disease with optical coherence tomographic angiography findings. JAMA Ophthalmol.
3. J. De Fauw (2018). Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med.
4. E.A. Swanson and J.G. Fujimoto (2017). The ecosystem that powered the translation of OCT from fundamental research to clinical and commercial impact. Biomed Opt Express, Vol. 8, Issue 3, pp. 1638-1664.
/Buyers_Guide/Wasatch_Photonics_Inc_Systems_Div/c20420
Published: September 2018
OCTopthalmicDeepMindMoorfields Eye HospitalFourier Domain OCTAIspectrometer sensorsCMOS technologySD-OCTFeatures