Replacement bones and blood vessels are just two types of artificial tissues that require deep-imaging microscopy techniques to develop.
Studying useful materials is not limited to macroscale structures such as sheet metal, optical glass, wood or concrete. Some of the most exciting work being done in materials research these days is on a much tinier scale and has applications that can get well under your skin.
Polyurethane is already a useful and ubiquitous material, found in common objects from skateboard wheels and Spandex to wood sealer and foam insulation. It also is found in many artificial body parts such as replacement hip joints – but while it is strong and lightweight enough for these applications, there is much room for improvement. Furthermore, interest in using polyurethane for even finer structures, such as artificial blood vessels and other replacement tissues, is growing.
Improved polyurethane-based “nanohybrids” will be very useful for biomedical applications, said Pralay Maiti, coordinator of the School of Materials Science and Technology at Banaras Hindu University’s Institute of Technology in Varanasi, India.
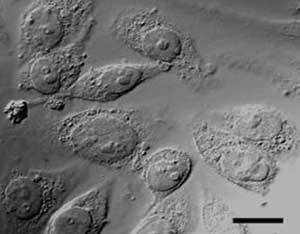
Differential interference contrast microscopy reveals the ability of cells to adhere to the nanohybrid substrate. Courtesy of the American Chemical Society.
These amalgamated materials under development – which can be compared with the doped crystals used in laser media and in optical glass – could be used to improve the strength, durability and flexibility of polyurethane-based biomaterials, and also to add controlled drug delivery to the list of tasks they can perform.
As with many polymers, polyurethane molecules have the capacity to assemble into long strands with no external influences. Such self-assembly results in some synergistic effects, lending them strength and toughness, Maiti said.
The formation of polymers through self-assembly, however, is still full of mystery, requiring careful analysis through multiple techniques. Some polymers employ hydrogen bonds to form stacks of molecule-thick sheets to become flat films useful for biomaterial engineering. These can be investigated using various techniques as they go through several stages of formation. For example, when polymers begin as molecular planes stacked together via hydrogen bonds, researchers use small-angle neutron scattering and x-ray diffractometry analysis. When one polymer progresses to its next stage of formation, atomic force microscopy becomes useful. When polymers at this stage begin to accumulate into tiny clusters discernible as crystallites, they can be viewed by optical microscopes.
Ultimately, improving polyurethane and similar polymers by “tuning” them with added materials will depend upon using the right analytical tool.
Turning polyurethane into a biomaterial useful for becoming substitute veins, arteries or other fine tissues will depend on adding dopants that not only increase strength and toughness, but also help the material hold onto living cells. The polymer is the scaffold upon which living tissues are hosted. The added material that Maiti’s group is focused on is a derivative of montmorillonite, or magnesium aluminum silicate, which the researchers call “nanoclay.”
The nanoclay substance is added prior to the two-step process used to create fresh batches of polyurethane. In the first step, a prepolymer material – plus nanoclay – is prepared. The second step, which controls strength and durability, is the extension of the linking polymeric chain via materials such as hydroquinone or biphenol.
“The main purpose of chain extension is the reaction of unreacted di-isocyanates with diols, which results in the higher molecular weight of the polyurethanes,” Maiti said. His group used a series of chain extenders to test their effect on the properties of the final polyurethane/ nanoclay development.
To examine the various versions of clay-infused polyurethane, Maiti and his colleagues turned to various microscopy techniques. They used a polarizing optical microscope made by Leitz (now part of Leica Microsystems of Wetzlar, Germany) to examine the sheet’s surface morphology, capturing its finely segmented structure. An atomic force microscope made by NT-MDT Co. of Moscow and set in tapping mode determined the domain structure of the sheets, indicating the size of hard-segmented zones created with the added nanoclay.
A scanning electron microscope from Tokyo-based Hitachi High-Technologies Corp. showed the surface morphology at higher magnification than an optical microscope provides, Maiti said, and x-ray and small-angle-neutron scattering techniques helped reveal the intricacies of layer spacing and of larger assemblies made up of several molecular sheets, respectively.
Lastly, the group used a transmission electron microscope made by FEI Co. of Hillsboro, Ore., to study the dispersion of two-dimensional nanoclays within the polyurethane molecular matrix.
“The unique feature of this work is that we could capture every possible step of the self-assembly phenomena, starting from molecular sheet (nanometer dimension) to bigger agglomerates (micron scale) using those imaging and scattering techniques,” Maiti said.
Building collagen scaffolds
Another major target of biomaterial engineers is collagen, the most abundant protein found in people. Collagen is a basic component of the extracellular matrix – the “backing board” that holds cells together in a swatch of tissue – and it possesses unique properties, including negligible cytotoxic response and ready availability – that make it widely used as biomaterial.
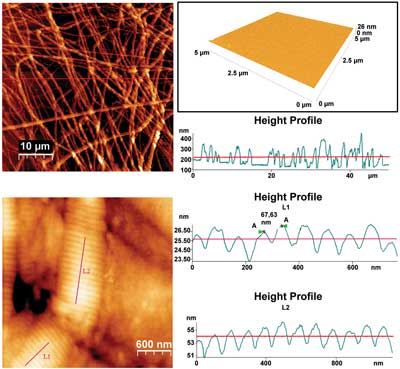
Top left: Collagen fibers formed on mica surface. Top right: The atomically
flat surface of a freshly cleaved mica substrate. Bottom: The D
band (67 and 100 nm) as it was imaged in collagen fibrils on mica.
Courtesy of Dido Yova, National Technical University of Athens.
“The value of collagen as biomaterial has led research on [its] use in scaffolds for ligament repair, collagen grafts for scar and burn repair, and the engineering of osteochondral tissue,” said Dido Yova, director of the Biomedical Optics and Applied Biophysics Laboratory at the National Technical University of Athens in Greece. As with polyurethane, collagen is a candidate material for repairing or replacing heart valves and bones.
To work well within the body, collagen, polyurethane and similar materials must be biocompatible and characterized from controllable processing conditions. They also must be robust and hydrophilic, which helps them support cell attachment.
Yova and her colleagues are working to form collagen-based biomaterials with an eye toward controlling surface characteristics, such as roughness and the size and orientation of collagen fibers.
“[Our] aim is to develop nanostructured collagen films, while the surface retains the bulk properties of collagen,” she said. “It is very challenging to understand and control the spatial organization of adsorbed protein layers, like collagen, in the nanoscale.”
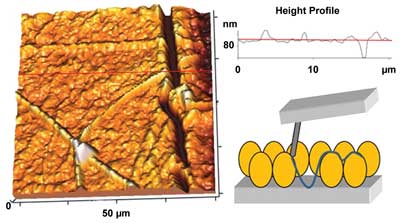
Left: A 3-D topographic image (20 x 20 µm via tapping-mode AFM) presenting the aging of PPS after six months, showing a crack 100 nm wide. Top right: The height profile of a single horizontal line from the left previous image. Bottom right: A schematic of how the AFM measures the height of the surface crack. Courtesy of Dido Yova.
Type I collagen, which is the most common fiberlike form of the material, consists of three amino acid chains that form rod-shaped triple helixes, which self-assemble into fibrils. Although it is largely self-assembling, it still is sensitive to the effects of cellular activities, particularly in young or healing tissues, Yova said. The complex structure of type I collagen presents as different morphologies in different tissues, yielding different functions. This complicates attempts to direct collagen formation as well as the design and creation of artificial structures.
To fully characterize the progression of collagen fibril and thin films as they form, Yova and her team chiefly use an atomic force microscope (AFM) made by Veeco (but manufactured since 2010 by Bruker Corp. of Billerica, Mass.).
Using an AFM for delicate materials research is common but presents its own set of setup issues (see sidebar below). Choosing the appropriate substrate is important as well, and this task greatly depends on the samples to be imaged. Rough surfaces are not useful as a substrate material; therefore, the most widely used materials are glass and mica – especially muscovite mica.
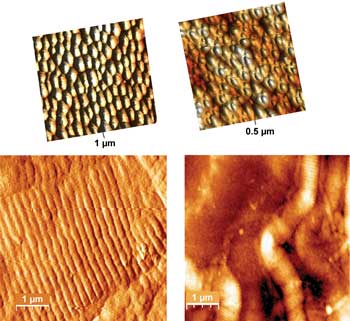
Topographic images of polystyrene particle surfaces (PPS) used as potential AFM substrate. On top are representations of the hexagonal packing of the particles. Courtesy of Dido Yova.
“Mica is one of the smoothest substrates, with a roughness of only ~0.1 nm, and the use of freshly cleaved mica provides a clean surface which does not demand a further method for removing contamination,” Yova said. Mica is readily available and inexpensive, as well as hydrophilic, which is desirable. By comparison, glass is more than three times as rough (0.3 to 0.5 nm). Silicon and highly ordered pyrolytic graphite (HOPG) also can be used. HOPG is very useful in some studies because it can be reused numerous times, Yova said, but it is hydrophobic and more costly than the other choices. In recent experiments, Yova’s group also tested polystyrene beads as a possible substrate material. Polystyrene is transparent, nontoxic, stable and inexpensive.
“AFM is a very powerful technique for studying biomaterials, since it provides high-resolution imaging of structure, combined with measurement of surface properties, combined with measurement of surface properties and surface-dependent intermolecular interactions under different conditions,” Yova said.
Her team continues to test various types of substrates and collagen deposition techniques to find the best ways to establish novel biomaterials from the nanoscale to the macroscale. Its ultimate goal is to fully clarify the roles played by various parameters and to determine the best characteristics for collagen-based biomaterials.
“Thin collagen films will be used to investigate cell-biomaterial interactions so as to correlate specific biomaterial nanocharacteristics with cells’ behavior,” she said.
A more useful plaque
Polyurethane is a practical and safe artificial substance, and collagen, a ubiquitous protein in mammalian structures, but less benign materials may also be directed toward helpfulness. Amyloid plaques are known for confounding neuronal signals in the brains of patients with Huntington’s, Parkinson’s and Alzheimer’s diseases. These plaques are composed of protein fibers, but recent research indicates that these fibers can be used to help shape novel biomaterial formation.
Whether naturally occurring or specifically designed, amyloid fibers are good candidates for making nanoscale biomaterials because they efficiently self-assemble into well-defined structures and are relatively inexpensive, said Juan José Valle-Delgado of the Institute for Bioengineering of Catalonia near Barcelona, Spain. Valle and his colleagues are using AFM and a technique called single-molecule force spectroscopy to suss out the best way to use amyloid fibers, or fibrils, to support new nanostructures.
AFM’s combination of high sensitivity and the ability to operate in liquid environments was attractive to Valle’s team for the characterization of the fibrils. The researchers considered transmission electron microscopy (TEM) as well, but that technique requires samples to be dried, which could affect the way in which the proteins change shape (conformation); it also could affect the final structure of the assembled fibril. Cryo-TEM can avoid that uncertainty, Valle said, but many images must be processed to obtain a high-resolution computer reconstruction.
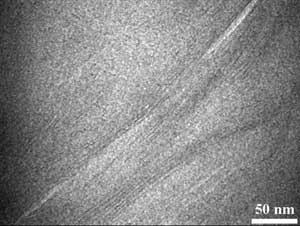
Transmission electron microscopy image of a polyurethane nanohybrid, showing the dispersion of two-dimensional nanoclay within the polyurethane matrix. Courtesy of the American Chemical Society.
With AFM, he noted, the tip geometry is an important factor that affects the resolution. Horizontal dimensions are usually overestimated in AFM images because of the tip geometry, but vertical dimensions are very precise, he added. In the case of soft samples, such as amyloid fibrils, scanning with the tip must be done very carefully to prevent damaging the sample, which is sometimes very tricky in liquid environments.
The investigators used a Veeco AFM to study the self-assembly of amyloid fibrils derived from the human peptide hormone amylin. Unlike Yova’s group, Valle and his colleagues chose HOPG as the appropriate substrate material. They cleaved the HOPG slab prior to depositing the sample to obtain the cleanest, smoothest surface possible.
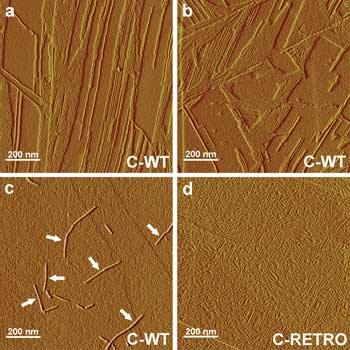
AFM images show self-assembled wild-type (C-WT) and reverse-sequence (C-RETRO) human amylin during formation of fibrils. Fibrils growing on top of a dense pack of other protofibrils are indicated by arrows (c). Courtesy of Soft Matter, a journal of the Royal Society of Chemistry.
“Highly oriented pyrolytic graphite is a quite smooth substrate,” Valle said. “However, unlike hydrophilic mica, HOPG is a hydrophobic substrate. The hydrophobic nature of HOPG could favor the adsorption of hydrophobic peptide aggregates.”
The group used the Veeco AFM to characterize the surface of the massing fibrils, but turned to a different instrument, made by Asylum Research of Santa Barbara, Calif., to analyze the force binding the peptides together as they lay on a hydrophilic mica substrate. “Force spectroscopy” or “single-molecule force spectroscopy” are names used in the AFM community for a technique that measures the binding forces between molecules of interest – for example, between an antibody and an antigen – or to analyze the mechanical properties of polymeric biomolecules such as proteins when they are stretched.

AFM images show real-time deposition of RETRO protofibrils. Courtesy of Soft Matter, a journal of the Royal Society of Chemistry.
Any AFM model can obtain both images and force measurements, but there is a subtle difference between the two instruments, Valle said.
“The Asylum Research AFM was preferred for force measurements because it is better designed to avoid drift when moving over the substrate to collect force curves [at] different points,” he said.
Both techniques showed the researchers that amyloid fibrils could be used as templates for nanoscale wires, with potential application as guides for nerve cell growth or as scaffolds for bone reconstruction. Future work by biomaterial researchers likely will be found in a wide range of replacement parts in everyone’s body.
Getting the most mileage out of atomic force microscopy
When using atomic force microscopy (AFM) to characterize novel materials, such as artificial biological tissues, there are a few logistical issues concerning instrument setup and sample preparation.
Dido Yova of the National Technical University of Athens in Greece said that sample preparation for AFM research is not complicated but noted that there are some crucially important steps to take:
The type of sample and its size are very important. Sample dimensions must be realistic because the majority of AFM stages put constraints on maximum sample size. For example, a typical AFM stage can hold a sample measuring 50 x 50 x 20 mm, while the maximum horizontal and vertical scan ranges are ~90 mm and 1 to 20 µm, respectively. AFM probes must be able to access sample features directly; e.g., noteworthy features located inside holes smaller than the AFM tip will not be reachable.
Samples must be rigidly mounted to the substrate. If the material is not rigidly adhered, the probe can move the sample material to the edge of the scan range and the image appears as though there is nothing on the surface. Furthermore, fragments from the material surface can become attached to the AFM probe, resulting in imaging artifacts.
Samples must be clean. If the surface is dirty, such as with a thick contamination layer, the AFM probe must penetrate the contamination layer to reach the surface, resulting in distortions in the final image.
Samples and their substrates must be mounted tightly to the AFM stage. Loose fittings will make the system more prone to vibrations that reduce the resolution of the microscope.