Full company details
Excelitas Technologies Corp.
 Corporate Headquarters
Corporate Headquarters
2545 Railroad St., Ste. 300
Pittsburgh, PA 15222
United States
Illumination Advancing Fluorescence Microscopy in Life Sciences, Medical Realms
BioPhotonics
Sep/Oct 2023LEDs, known for their long lifetimes, provide versatility to bring fluorescence techniques into specialties such as in situ hybridization, digital pathology, and surgical guidance.KAVITA ASWANI, EXCELITAS TECHNOLOGIES CORP.
In many academic research and medical settings, microscopy and imaging have transitioned from using traditional lamp illumination, such as mercury and Xenon lamphouses, to solid-state LED technologies. The benefits of moving to LEDs include long lifetimes and increased stability of the light source, eliminating the need to replace or dispose of toxic bulb waste. Fluorescence microscopy, for its part, has traditionally used and been limited by the spectrum of the mercury arc lamp, which has defined the chemistry of fluorophores, as well as the excitation and emission filters used in fluorescence imaging across the world. The discrete peaks of the mercury arc lamp spectrum have dictated the chemistry of the most common fluorophores, including DAPI, FITC, TRITC, and cyanine dyes. These peaks have also led to the creation of genetic constructs of fluorescent proteins, which have been developed and used for decades in live-cell microscopy.
While the transition to LED technology provides many benefits, users and manufacturers of instrumentation in fluorescence microscopy must account for the differences in the peak optical power between lamps and LEDs and must optimize their filters to achieve maximum excitation efficiency to image their fluorophores.
Fluorescence microscopy basics
Fluorescence microscopy is a technique employed in biology, chemistry, and other scientific fields to visualize and study various samples (Figure 1). It involves the use of fluorescent molecules, also known as fluorophores, which emit light of a specific wavelength when excited by the light of a specific higher-energy wavelength (Figure 2). The basic principle behind fluorescence microscopy is the absorption of light or photons by a fluorophore, which causes it to transition to an excited state. The fluorophore returns to its ground state, releasing energy in the process in the form of light (Figure 3). This emitted light is typically a longer/higher wavelength than the excitation light, allowing it to be easily distinguished from the background and detected using the naked eye, a camera, or other sensor.
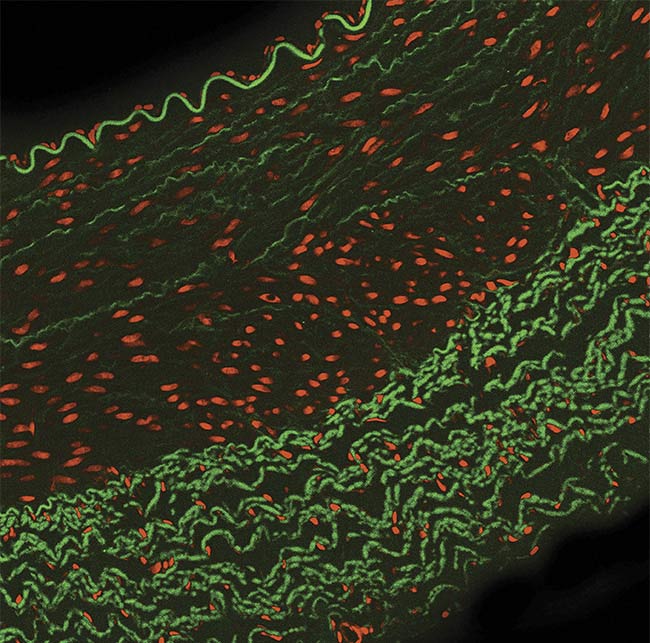
Figure 1. A typical multilabeled fluorescence image. Courtesy of Excelitas.
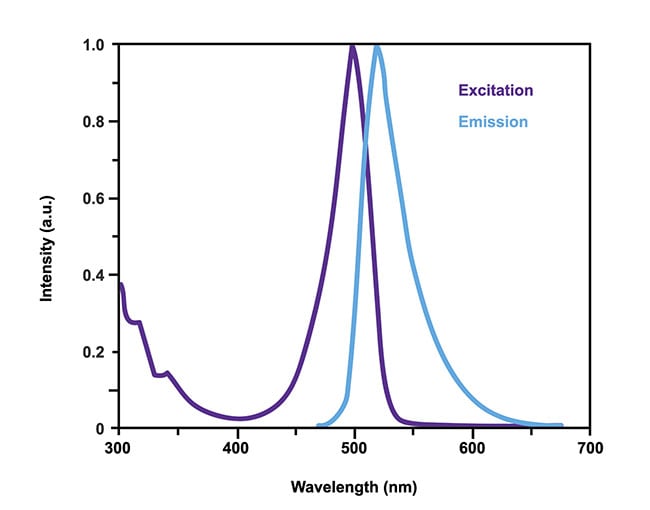
Figure 2. An example of the excitation and emission spectrum of a fluorophore. The light emitted is
usually of a higher wavelength than that of the excitation. Courtesy of Excelitas.
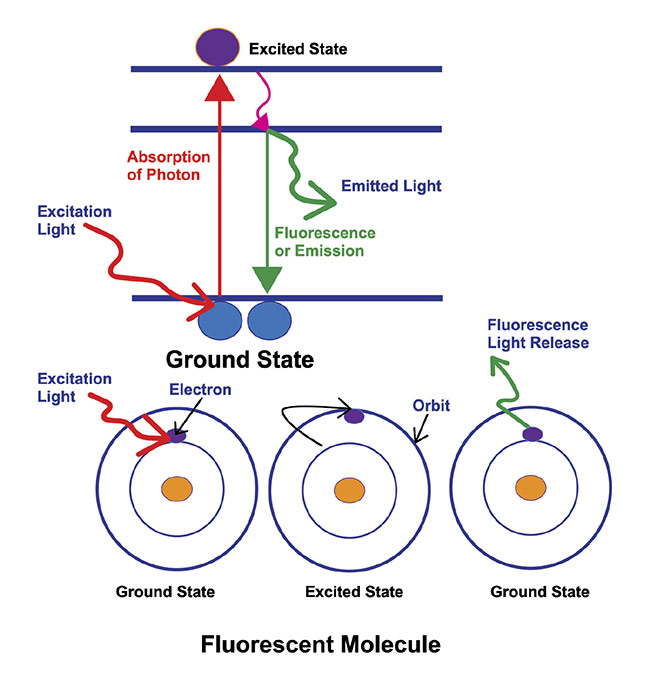
Figure 3. An illustration of the basic principle behind fluorescence microscopy. The absorption of light or photons by a fluorophore, which causes it to transition to an excited state. The fluorophore returns to its ground state, releasing energy in the process, in the form of light. Courtesy of Excelitas.
In practice during life sciences research, a sample of interest is labeled with specific fluorophores that selectively bind to or interact with certain cellular structures, molecules, or proteins. This labeling enables researchers to visualize and study the distribution, localization, and dynamics of these targets within the sample, providing, for example, information that is critical to studying proteins and how they function in normal cells versus tumor cells.
Fluorescence microscopy offers several advantages in comparison with traditional microscopy techniques for the detailed examination of a sample. It allows for high sensitivity and specificity, because fluorophores can be designed to bind to particular targets. Extra specificity can be achieved by programming a gene so that when it expresses a protein of interest, the protein is intrinsically tagged with fluorescence, also known as a fluorescent protein. The latter allows for greater specificity of labeling the target, without the outside interference of adding an external fluorophore to the sample — which could potentially alter or compromise the functioning of the sample — as well as the behavior one wishes to study for research or medical purposes.
Fluorescence labeling also permits the simultaneous detection of multiple fluorophores with distinct emission wavelengths, enabling the visualization of multiple targets or biomarkers within a single sample. Additionally, fluorescence microscopy can be combined with other techniques, such as live-cell imaging and super-resolution microscopy, to provide detailed insights into the mechanics of cellular processes and structures.
Overall, fluorescence microscopy has become an indispensable tool in biological research, aiding in the study of cellular and molecular biology, immunology, neuroscience, and many other areas of biological investigation.
Advantages of LEDs
LEDs initially found application in industries such as electronic displays and indicator lights. But their potential in microscopy was ultimately recognized due to their small size, low power consumption, precise control, long lifetime, and ability to emit light of different colors.
In the 1990s, LEDs began to be integrated into microscopes as brightfield light sources in both research and industry applications
1. They were also employed in darkfield, phase contrast, and differential interference contrast microscopy, among others. In the 2000s, LEDs were brought into the world of fluorescence microscopy
2, where they provided scientists the advantages of bright and stable illumination, long lifetimes (typically tens of thousands of hours), energy efficiency, narrowband light, and rapid switching between wavelengths. The ability to switch between multiple LED wavelengths made them useful in multichannel fluorescence imaging. Additionally, LEDs generated less heat compared to traditional light sources, reducing the risk of specimen damage and fluorophore bleaching. LEDs also played a crucial role in the integration of microscopy with digital imaging systems for both digital pathology and slide scanning. Their stable and controllable illumination facilitated the capture of high-quality images and videos for documentation, analysis, and publication.
However, the integration of LEDs into fluorescence microscopy was an uphill battle for system designers. When LED sources first became available in the microscopy world, they had low optical power and a limited range of wavelengths, preventing early adoption for detailed sample research. Today, almost two decades later, advancements in the semiconductor industry have enhanced LEDs, allowing them to replace lamps used in microscopy and fluorescence imaging. These innovations have, in turn, significantly contributed to advancements in research as well as clinical applications of LEDs and fluorescence microscopy.
LEDs allow more control
LEDs have much longer lifetimes than traditional light sources, such as incandescent bulbs, mercury, xenon, or halogen lamps. Their extended operational lifespan reduces the need for bulb stocking and frequent replacements, making microscopy more cost-effective and efficient in laboratory settings. In addition, LEDs provide an opportunity to streamline and simplify microscopy hardware by reducing or even eliminating the need for filter wheels and filter cubes used in fluorescence microscopy. Selecting LEDs with different wavelengths eliminates the need for the movement of mechanical filters to switch between excitation wavelengths to target fluorophores of interest, allowing for faster and more precise multichannel imaging. Precise control of intensity and timing enables researchers to optimize imaging conditions. This control is crucial for time-lapse imaging, high-speed multiplex imaging, and live or dynamic experiments.
LEDs have become a standard light source in many microscopy setups. They continue to advance, with improvements in brightness, spectral range, and stability that has driven their use in fluorescence microscopy.
Research applications
Fluorescence microscopy is a standard tool used in biological research. Labeling various biological markers with fluorescent proteins or fluorescent dyes allows localization and comparisons of normal and diseased states. This provides researchers with valuable information on the change in proteins expressed in diseases, leading to insights into the cause of the disease and targets for potential treatments.
There are several techniques used to study molecular interactions to learn more about diseases. Förster resonance energy transfer uses fluorescence to measure the distance between proteins to determine whether they are close enough to interact. Optogenetics uses light to turn genes on and off, and fluorescence in situ hybridization helps to map out genes and detect genetic alterations. Each fluorescence technique uses specific properties of light, and therefore, the light sources chosen for these studies are crucial in determining the success of generating useful data.
Uses in pathology
Pathology is the identification of disease in bodily fluids, cells, or tissue. Traditionally, pathologists have used brightfield dyes to stain a sample and have relied on their expertise to identify disease-causing structures or abnormalities in the sample when compared with healthy tissue. Aside from disease diagnosis, some pathologists specialize in research, investigating the mechanisms of diseases, developing new diagnostic tests, and exploring therapeutic approaches.
Fluorescence in pathology includes the exploitation of immunofluorescence and fluorescence in situ hybridization as described above. Digital pathology is the science of scanning hundreds or thousands of slides, taking images of the staining patterns or fluorescence, and digitizing the data for analysis, storage, and record keeping. This also allows for remote analysis of the slides and even collaboration between pathologists for a second opinion, which benefits all specialists.
Surgical guidance
Fluorescence imaging techniques, and specifically fluorescence-guided surgery, are becoming more common for assisting the surgeon with the identification and resection of tumors. Special dyes or contrast agents that selectively accumulate in certain cell types can be administered to patients before or during surgery. For example, indocyanine green (ICG) labels blood plasma and protoporphyrin IX (PPIX) used with 5-aminolevulinic acid is used for tumor labeling. When exposed to specific wavelengths of light, these dyes emit fluorescence, enabling surgeons to visualize and precisely remove tumor cells while sparing adjacent healthy tissue and vasculature.
Choosing the light source
The key in any of these applications described above is choosing the right light source. Without appropriate illumination or excitation and a good detector or camera, the data generated is of no quantitative significance.
Key factors to consider are:
•
Stability and repeatability: All light sources, whether lamp, LED, or laser, will degrade in optical power over time. Stability is important as lamp flicker or laser speckle can interfere with data integrity. Repeatability ensures that the same dose of light reaches the sample with each image captured in an experiment or procedure.
•
Uniformity: When imaging a slide, uniformity across the field of view is crucial to ensure that the same amount of light is reaching each part of the slide to consistently excite biomarkers in different areas of the sample (Figure 4). This becomes more evident when whole slides are scanned using tiling and stitching several images together.
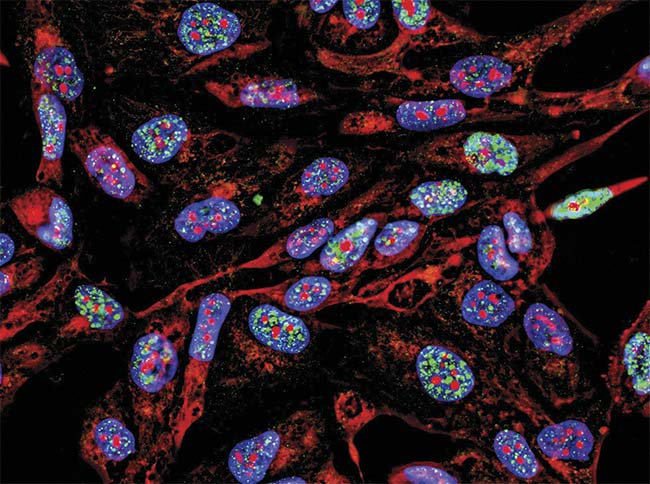
Figure 4. A uniform light source will generate accurate data representative of the sample, in this case, fluorescence imaging immunofluorescence of cancer cells growing in 2D with nuclei (blue), cytoplasm (red), and DNA damage foci (green). Courtesy of iStock.com/Nicola Ferrari.
•
Wavelengths: Each fluorophore or fluorescent protein has an excitation wavelength, which needs to be present in the light source for efficient fluorophore excitation. If the researcher wants to perform ratiometric calcium imaging using Fura-2, they will need 340- and 380-nm wavelengths at the same optical power to record the images at both wavelengths after adding the calcium indicator (Figure 5). Traditionally, xenon lamps were the only option for Fura-2 imaging due to their fairly uniform spectrum, which allows for the two images to be captured at the same optical intensity. Similarly, if the surgeon wants to excite ICG, they will need an ~800-nm wavelength to be able to visualize the plasma or blood during their surgery.

Figure 5. Capsaicin elicits calcium responses in rat (dorsal root ganglion (DRG)) sensory neurons. Phase-contrast (left) and ratiometric images of Fura-2 loaded DRG sensory neurons. 300-nm capsaicin (TRPV1 agonist) elicited calcium responses in ~50% of sensory neurons. Adapted with permission from Reference 3.
•
Optical Power: The amount of power required to excite a fluorophore depends on its excitation efficiency and the required speed of acquisition of the image. The first step is to optimize the staining technique to determine the appropriate amount of stain for the antibodies and cell line being used. Faster acquisition of images using a high-power light source must be balanced with photobleaching and phototoxic effects of the light.
•
Lifetime: The lifetime of lamps is typically a few hundred hours, requiring the stocking of spare lamps, replacement, and eventually disposal. LEDs usually last for tens of thousands of hours. This becomes especially important in slide scanning systems, which perform imaging for several hours a day and cannot afford downtime for frequent bulb replacement.
•
Switching: A broadband white light source is usually sufficient for most applications as the filter wheel will select the appropriate wavelengths for fluorophore excitation. For more challenging applications in which higher speed is required, or individual control of wavelengths is needed (as in the case of Fura-2 imaging), a switchable light source works best. High-throughput slide scanners will also typically use a fast-switching light source to speed up imaging of thousands of samples/fluorophores.
Overcoming the ‘Green Gap’
Traditional lamp technologies have rich broadband spectra. Matching this range and intensity of wavelengths is one of the biggest challenges for LEDs.
In particular, light source manufacturers have been challenged with designing fluorescence illumination systems that cover the so-called Green Gap. The most common fluorophores were designed using the spectral peaks of mercury lamps. LEDs initially struggled with exciting fluorophores in a gap between 540 and 590 nm, which corresponds with visible green wavelengths. Manufacturers have generated solutions to address this gap using LED arrays, wavelength phosphor conversion technology, and laser solutions.
As an example, Excelitas Technologies Corp.’s Laser LED Hybrid Drive uses a laser phosphor conversion technique to generate high-power light in the 540- to 590-nm region. X-Cite systems employing this technique account for all thermal, electrical, and optical parameters to maximize light conversion and delivery of light to the sample.
This technology has the ability to be more efficient than other phosphor conversion techniques and generate enough optical power to rival a mercury arc lamp.
Looking to the future
The use of LEDs in microscopy (research and medical) has revolutionized the field by providing numerous advantages over traditional light sources. And there will be further improvements and developments of this technology to look forward to.
LED technology advancements are leading to brighter and higher luminous chips. Higher efficiency leads to low heat generation and less power consumption, making them more environmentally friendly with longer lifetimes.
Just as Fura-2 and ICG traditionally relied on lamps and can now employ LEDs for fluorescence excitation, with newer LEDs covering wavelengths further into the UV and IR ranges, applications that traditionally have used laser technologies are bound to transition to LEDs in many cases. Additionally, the spectral bandwidth will get narrower, eliminating the need for filters in multiplex fluorescence experiments. Alternatively, there could be ultracompact LEDs capable of producing several wavelengths simultaneously, allowing for rapid multicolor fluorescence imaging with one single light source.
LEDs combined with adaptive optics, such as custom lenses and mirrors, among others, could enable their entry into super-resolution or light sheet microscopy. Further miniaturization of LED assemblies and cooling systems will enable them to be more cost-effective and portable for remote use, or used by researchers with limited funds.
Continued advancement in LEDs and associated technologies will enhance the portability of microscopy systems, system versatility, and imaging capabilities. Imaging at nanometer resolution can shed light on the details of cellular processes and interactions, allowing a deeper understanding of molecular biology and mechanisms of diseases. Integration of fluorescence microscopy with electron microscopy will provide a more holistic understanding of complex systems. Improved light sources will also speed up imaging, accelerating drug discovery and genetic screening for diseases.
Fluorescence microscopy holds great potential for revolutionizing various scientific disciplines and medical applications. With continued advancements in technology, researchers will be able to gain new insights into complex biological processes, advancing the understanding of health and disease, and ultimately leading to improved medical treatments and diagnostics.
Meet the author
Kavita Aswani is the senior applications scientist for biomedical products at Excelitas Technologies. With a doctorate in anatomy and cell biology earned from the University of Iowa, she boasts an extensive background, encompassing seven years of scientific research expertise and over 23 years of applications experience in the field of microscopy and the fluorescence industry. Throughout her career, Kavita has contributed to numerous publications and peer-reviewed journal articles. Her specialized areas of work include wide-field illumination microscopy, laser scanning microscopy, and flow cytometry. She remains an engaged member of both the Society for Neuroscience and the Royal Microscopical Society; email:
[email protected].
References
1. J.M. Beach. (1997). A LED light calibration source for dual-wavelength microscopy.
Cell Calcium, Vol. 21, No. 1, pp. 63-68.
2. S.J. Hart and R.D. JiJi. (2002). Light emitting diode excitation emission matrix fluorescence microscopy.
Analyst, Vol. 127, No. 12, pp. 1693-1699.
3. D. Liu et al. (2021). X-Cite fluorescence illumination in control, application report,
https://www.excelitas.com/sites/default/files/2021-04/XC_X-Cite%20NOVEM%20App-Note.pdf.