Optical coherence tomography is being used to image not only the eyes but other areas of the bodies of animals in veterinary offices, pinpointing and mapping data as in human applications.
Medical doctors have long understood the value of optical coherence tomography (OCT) for monitoring human health. Veterinarians are learning that the technology can be just as vital in evaluating the well-being of patients in the animal kingdom. Often used in conjunction with other technologies, OCT has the capacity to map out tissue at high resolution as a reference point for further experiments and treatments.
OCT uses low-coherence, backscattered light to create cross-sectional images of tissue structure at the micron scale, and it performs this function quickly. It works similarly to ultrasound except that it uses light instead of sound, and in the end, the picture is clearer.
The technology is recognized as a mechanism to resolve images of deterioration and treatment progression in the eyes of both human and nonhuman animals (Figure 1), and it has also been adapted to investigate cancerous tissue and joint strength in particular species. The nonhuman subjects of study and examination by veterinarians using OCT vary from the family pet to animals that are to be returned to the wild.
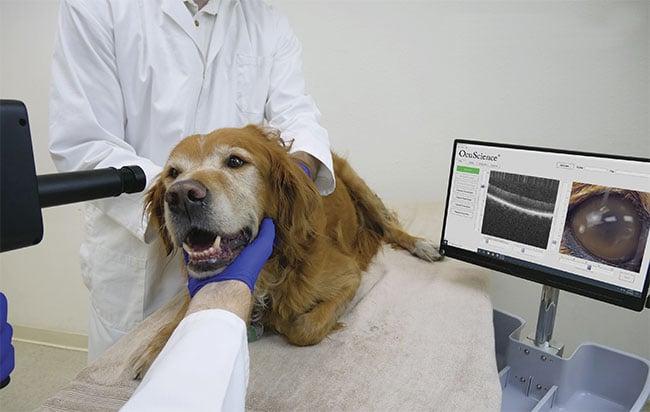
Figure 1. A dog’s eyes are examined with an optical coherence tomography (OCT) system. Courtesy of OcuScience.
Canine sarcoma
Like many veterinarians and other medical specialists, Laura Selmic has performed surgery on cats, dogs, and various small animals to treat and remove cancer from their bodies. She said she ultimately embraced OCT as a mechanism to improve the precision — and hence the success — of such surgery (Figure 2).
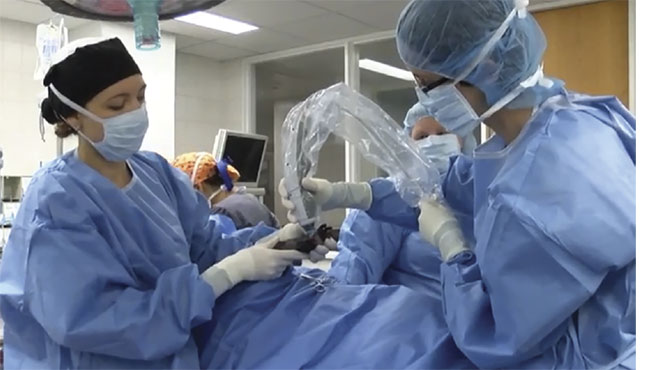
Figure 2. A veterinary surgical team uses OCT to intraoperatively image a patient. Courtesy of
Laura Selmic.
“We have studied animals in vivo and ex vivo,” said Selmic, who is a veterinary surgeon and an associate professor at The Ohio State University College of Veterinary Medicine. “We always want to make sure we remove all the cancer, and [that] none remains.”
She said her research groups have worked mostly with a hand-held OCT device, which was initially custom-built by colleague Stephen Boppart, the Abel Bliss Professor of Engineering and director of imaging at the University of Illinois at Urbana-Champaign. In more recent research, Selmic’s group used a commercially designed system.
In a study published last year, she and her team used a Bioptigen Inc.-designed spectral domain OCT (SD-OCT) system, which generates depth-resolved tissue structure information from the intensity and delay of backscattered light via spectral analysis. The system had a central wavelength of 1310 nm and an illumination power of ~5 mW, and resolution was 8 µm axially and 10 µm laterally1.
The team’s goal was to evaluate normal and abnormal histological characteristics in canines suffering from soft tissue sarcoma. The researchers were able to distinguish between adipose, skeletal, and sarcoma tissue based on the inherent scattering (Figure 3), which helped to identify margins of the area to be removed.
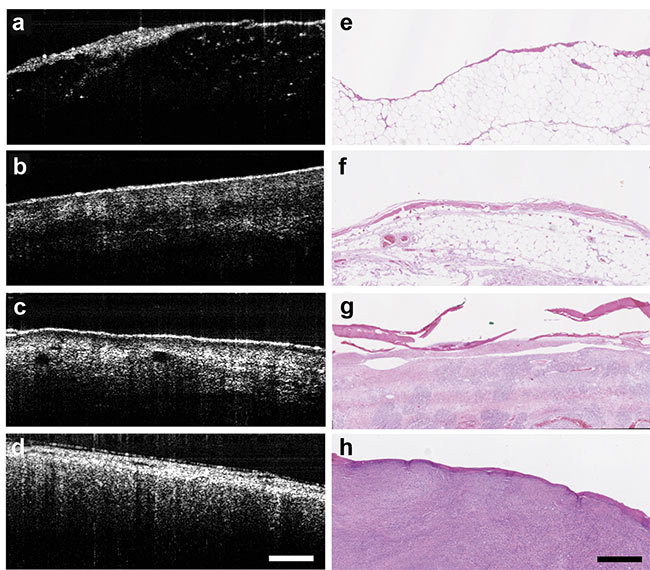
Figure 3. With the aid of an OCT system, tissue is differentiated between fat (a, e); muscle (b, f);
skin (c, g); and sarcoma (d, h). Courtesy of Laura Selmic.
“We found good accuracy with our system,” Selmic said. “With our work, we concentrate on finding a related application at the point of care, but we are able to provide useful information and collaborate with teams and researchers in pathology and radiology as well.”
She said in the future her team will work on devising an artificial intelligence (AI) algorithm to help clinicians differentiate between tissue types with accuracy.
A bird’s eye
Elsewhere, researchers have used SD-OCT technology to examine the eyes of various avian species that are hard to explore using other optical methods. Panagiotis Azmanis, a veterinary doctor at Dubai Falcon Hospital/Wadi Al Safa Wildlife Center in Dubai, United Arab Emirates, said while directed ophthalmoscopy only images the surface of the eye, OCT can map out the inner structure or distribution of any phyical changes.
With SD-OCT, depth-resolved structural information about tissue can be gathered by measuring the intensity and delay of backscattered light via spectral analysis of the interference pattern.
Azmanis has collaborated with other researchers in studies of this nature using SD-OCT on birds. He took part in research initiated by Franziska Rauscher, a vision scientist, and Mike Francke, a retinal biologist — both from Leipzig University Hospital in Germany. They determined that eye conditions in birds were often poorly diagnosed using other technology due to most birds’ small size.
In studies over the last several years, Rauscher said she and her colleagues have used the SPECTRALIS SD-OCT platform produced by Heidelberg Engineering, which provided data in high resolution.
“This is due to the eye tracker implemented into the system, which is key when imaging animal eyes, as they do not help by fixating the target,” she said, adding her team does not use custom-made components.
In one study, the researchers used SD-OCT to image the eyes of 39 birds and compared the results with those from ophthalmoscopy exams. Sixteen of the birds were found to have eye abnormalities that the system detected, many of which had resulted from blunt trauma. This contrasted with only five abnormalities identified by direct ophthalmoscopy. The equipment, which included a light source and a mirror with spectral patterns captured by a scanner, was set up to acquire line scans, star-shaped line scans, and volume scans2. A line scan is a row of pixels that captures data quickly, a star-shaped line scan measures data at the intersection of line scans, and a volume scan moves the line up and down the area being viewed.
“My choice of scan is the volume scan, but because the animal moves, it is best to obtain line scans first, alongside the infrared image acquired at the same time, to examine the area of interest in a speedy fashion,” Rauscher said. “A star-shaped scan is merely a few line scans. Once an area of interest is identified, a volume scan can be carried out there to obtain deeper information.”
To further develop SD-OCT technology for the future, she said eye models for specific kinds of birds would need to be integrated into a database, particularly since most OCT technology has been designed based on the optics of the human eye.
“We need to have at least corneal curvature for the animals we are measuring in order to obtain correct sizes at retinal level,” Rauscher said. “A landmark development would be to obtain correct biometry of the living eye in various species.”
Horse joints
Other researchers have followed a different avenue for putting OCT to use for the welfare of animals. A partnership involving the Department of Clinical Sciences at Utrecht University in the Netherlands and the Biophysics of Bone and Cartilage group at the University of Eastern Finland investigated the use of arthroscopic OCT in horses, and they experienced encouraging results.
Nikae te Moller, a postdoctoral researcher in the Department of Clinical Sciences at Utrecht, said the team’s work began in 2011 when they sought to determine whether intra-articular OCT could identify cartilage lesions in equine joints.
“Progressive degeneration of cartilage is a well-known hallmark of osteoarthritis [OA], a joint disorder that not only affects millions of people worldwide, but is also a common burden in animals,” she said. “The high incidence of OA is of particular concern in the horse industry, where it causes lameness and early retirements from the sport. Quantification of cartilage defects is important in order to predict their long-term progress and to be able to develop tailored, evidence-based repair strategies.”
In one study, the team used intra-articular OCT to verify cartilage thickness and thus the speed of sound within the cartilage in 94 samples. Earlier studies had shown that the speed of sound is slower in osteoarthritic cartilage than in healthy cartilage. Researchers used an OCT device attached to a thin catheter. The OCT technology captured cross-sectional images of the tissue (Figure 4). In the images, calcified cartilage was distinguishable from noncalcified cartilage and bone in virtually all the samples in question. Measurements were similar to those obtained via light microscopy3.
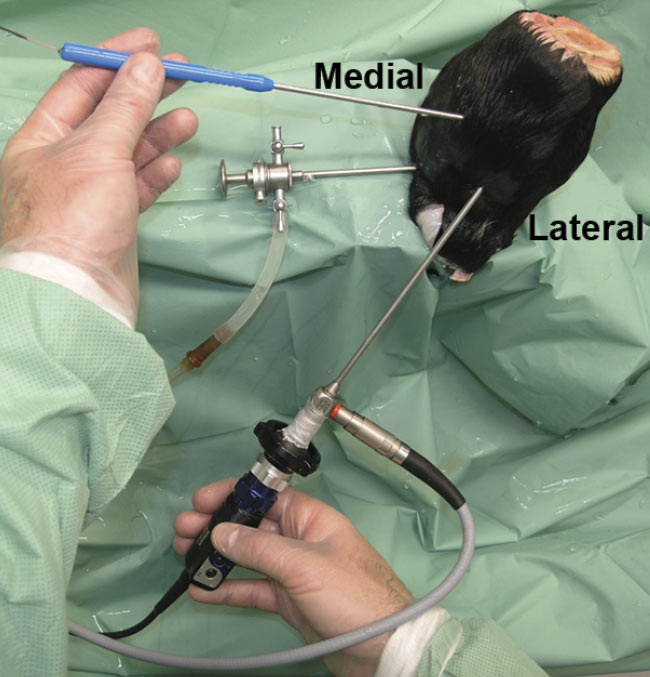
Figure 4. OCT arthroscopy imaging of an equine metacarpophalangeal (MCP) joint. A standard portal is used for the arthroscope, and a dorsomedial portal is used for the OCT catheter. Adapted with permission from Reference 3.
The researchers concluded that articular cartilage thicknesses below 1.8 mm were successfully measured with OCT and that this information could be used for detecting cartilage thinning and estimating lesion depth (Figure 5). According to te Moller, OCT proved to be superior to traditional arthroscopy, which is unable to track changes below a seemingly intact surface, and that MRI and CT lack the resolution needed to examine minute changes over time.
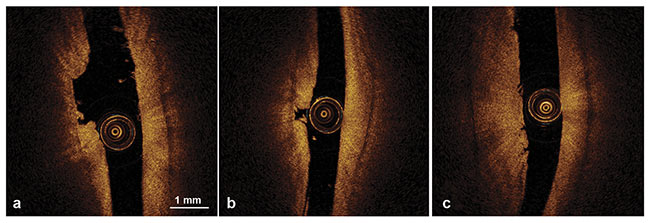
Figure 5. OCT morphological characteristics of articular cartilage lesions showing ulceration (a), ulceration and cartilage fragmentation (b), and superficial vertical clefts (c). Adapted with permission from Reference 3.
“We have combined intra-articular OCT with other techniques, including ultrasound and near-infrared spectroscopy (NIRS) to overcome its limited penetration depth (1.5 to 2 mm in cartilage) and to strengthen the quantitative character of the data that can be obtained,” she said. “The use of OCT and NIRS is of particular high potential: The combination of high-
resolution imaging (OCT) and the prediction of the surrounding cartilage properties (based on NIRS) can accurately assess the severity of damage and the degree of tissue degeneration extending from an injury.”
Rehabilitating turtles
In some cases, OCT technology can answer riddles found under the sea, such as in the case of an analysis of ocular changes in Kemp’s ridley sea turtles undergoing rehabilitation at the New England Aquarium in Boston a few years ago.
This rare species of sea turtle is critically endangered. Researchers involved in the study said many of these turtles in the northern Atlantic Ocean were failing to migrate to the Gulf of Mexico when the weather grew colder, effectively trapping themselves in a colder climate, which could lead to a variety of health problems ranging from metabolic dysfunction to ailments in the digestive and nervous systems.
The turtles may develop ocular disease as well. Keratopathy (damage to the cornea, often caused by loss of fluid or incomplete closure of the eyelid) and cataracts are common. But no baseline of information was available related to corneal thickness for the turtles4, said the team in their study.
“In some cases, ultrasound can be used to examine the eyes, but it doesn’t get the resolution of OCT, especially with the small, beady eyes these turtles have,” said study participant Christopher Pirie, a veterinary doctor and associate professor in small animal clinical sciences in the College of Veterinary Medicine at Michigan State University. “And the depth of penetration might not be as effective in getting the detail we need.”
The turtles were gently held in position while the OCT measurements were captured with an SD-OCT machine that had a special attachment. Three scans were collected, each starting from the center of the pupil. Total corneal thickness (TCT), epithelial thickness (ET), stromal thickness (ST), anterior chamber depth (ACD), and intraocular pressure (IOP) were measured. IOP was collected via rebound tonometry, which analyzes motion parameters (the rate of deceleration) of a bouncing probe after the probe makes contact with the cornea. A mean range was established for each measurement. For example, ~288 µm was recorded for TCT4.
“As you move the OCT instrument closer, you can focus the view and get a cross section of the eye,” Pirie said. “In some cases, the animals may have a shrunken globe. This will give a reference point for future studies.”
Branching out
As OCT technology has matured, scientists have gained new tools in their arsenal, Pirie said, such as OCT angiography (OCT-A). This modality uses laser light reflectance from moving red blood cells to depict blood vessels in the eye. Scans are taken repeatedly to track retinal changes.
While the technique requires no dye, Pirie’s team used an angiographic dye that helps them follow the drainage pathways.
“We have recently been using OCT-A to track retinal changes, and correlated them with information from angiograms,” he said. “This is a technique that is used a lot for humans with glaucoma, and while not a lot has been done with live animals, we have used this in dogs’ eyes and we hope to develop a better understanding of how these systems work.”
References
1. L. Selmic et al. (2019). Intra-operative
imaging of surgical margins of canine soft tissue sarcoma using optical coherence tomography. Vet Comp Oncol, Vol. 17,
Issue 1, pp. 80-88.
2. P. Azmanis et al. (2015). The additional
diagnostic value of optical coherence
tomography (OCT) and its application
procedure in a wide variety of avian
species. J Clin Exp Ophthalmol, Vol. 6, Issue 3, www.dx.doi.org/10.4172/2155-9570.1000431.
3. P. Puhakka et al. (2016). Optical coherence tomography enables accurate measurement of equine cartilage thickness for determination of speed of sound. Acta Orthop, Vol. 87, Issue 4, pp. 418-424.
4. K. Gornik et al. (2016). Ophthalmic variables in rehabilitated juvenile Kemp’s ridley sea turtles (Lepidochelys kempii). J Am Vet, Vol. 248, Issue 6, pp. 673-680.
Lining Up OCT Equipment
Given all the potential uses and settings in which OCT equipment can be administered, Daniel Lindgren, president of OcuScience, said the capability to work with various species must be designed into a desktop system for laboratory animals (Figure 1) and on a mobile cart for the animal hospital out in the field. In the lab, gas anesthesia delivery is built in, along with a scanner on a gimbal that pivots in front of the eye to enable anterior-to-posterior imaging of various animals.
Figure 1. A rabbit’s eyes are tested using an OCT instrument. Courtesy of OcuScience.
The company produces OCT/fundus cameras that capture images of the eyes of animals, from rodents and companion animals to horses (Figure 2). Lindgren said the company’s systems are designed with a sub-2-µm resolution.
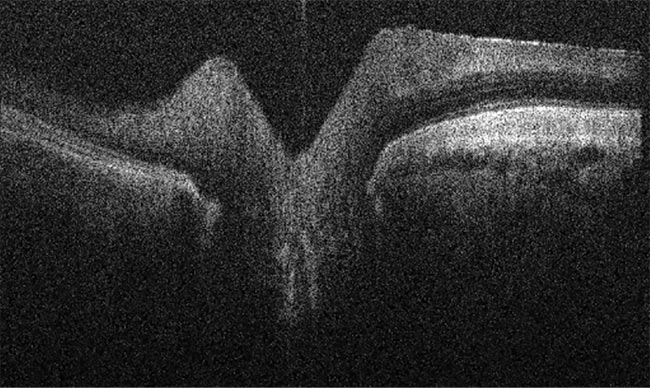
Figure 2. An OCT image of a horse retina. Courtesy of OcuScience.
“You can look into the eye using the fundus camera and acquire the OCT images,” he said. “It’s like looking at histology slides through a microscope, but in a living patient.”
The equipment needs to be adaptable because the intended patients don’t respond to instructions in most cases. “Our patients aren’t compliant and won’t put their chins on the chin rest and fixate on a target,” he said. “In fact, most don’t have chins, and given the chance, will run off.”
In the future, Lindgren said he hopes to develop various ways to analyze data from the corneas and retinas of different species, using the same features seen in human-based OCT.
“We need to be able to appreciate anatomical and physiological differences between species,” he said. “If we can establish a database for sharing this information, it will be easier to read, interpret, and understand the data for research and clinical care for all animals.”