Spectroscopy used to study nanoparticles, DNA and bacteria.
Jay Jeong, Newport Corp., and Dr. Alexei Gusev, Ultrafast Systems LLC
The interdisciplinary study of molecular science aims to expand our knowledge of everything around us, from the molecular bonds that give plastic widgets their strength to the electron transitions that power microprocessors, lasers and society as a whole. Physics, chemistry and biology have spent their entire histories pushing toward greater understanding of the fundamental laws that govern matter and energy, and it is hard to be more basic — more fundamental — than interactions at the atomic and molecular levels.
Studying these building blocks is difficult for a number of reasons. They are spatially very small, making direct observation difficult. Indirect observation, through spectroscopy and other methods, requires extremely high precision equipment. In the case of time-resolved absorption spectroscopy, studying material properties requires precise control of excitation and probe light sources across a broad spectrum as well as sophisticated data-acquisition software and hardware. Finally, molecular and atomic transitions can occur very quickly — on the femtosecond time scale — so the instrumentation must be able to keep up.

A transient absorption spectrometer is useful for studying transient ultrafast molecular events.
In recent years, ultrafast lasers with pulses as short as 10 fs, such as the Ti:sapphire, have provided chemists, biologists and physicists with the means to probe the dynamics of molecular interactions. However, spectroscopic data collection instrumentation has lagged. The result has been incomplete and/or noisy transient absorption spectra — puzzles with missing pieces.
Today turnkey CCD-based spectrometer designs with innovative control electronics and software allow scientists to excite and probe molecules as well as to collect data on excited states as fast as several kilohertz — without missing a single laser pulse. When combined with new levels of system integration, a white light continuum probe kit, an optical parametric amplifier excitation kit, a harmonic-generation kit, optimized 3-D visualization software and enhanced continuum generation out to 1600 nm, a system such as the Helios IR spectrometer manufactured by Newport Corp. gives researchers a complete view of ultrafast molecular transient events down to the femtosecond scale.

A sample is being excited by the spectrometer.
Photobiology studies
Ultrafast transitions happen in a broad variety of scientific explorations. For example, transient absorption spectroscopy studies dynamic events involving subjects from nanoparticles1 to DNA2 to bacteria.3 Several groups have used the technique to study systems from photophysics to photobiology, including cutting-edge research into DNA and laws governing cellular health.
Professor Harry A. Frank of the University of Connecticut in Storrs studied the dynamics of certain types of molecules, specifically, the color pigments found in lobsters. The organism’s red pigment comes from the carotenoid astaxanthin, and the blue pigment in lobster shells comes from crustacyanin, which is astaxanthin clumped together with a protein. The blue results from the protein pulling astaxanthin molecules close to each other.
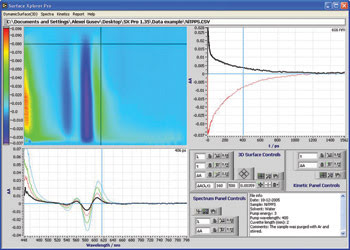
The system has an easy-to-use software interface.
In blue lobsters, a genetic mutation has caused an overabundance of the astaxanthin-wrapping proteins, tying up all of the red astaxanthin into blue crustacyanin. With a spectral range of 450 to 1600 nm and time resolution of approximately 100 fs, Frank’s group used the Helios to make transient absorption measurements on astaxanthin in CS2, methanol, acetonitrile solution and in α-crustacyanin. The measurements determined that the S1 state has a lifetime of approximately 5 ps, independent of solvent. In α-crustacyanin, the lifetime is 1.8 ps.4 The lifetime of the S2 state is measured to be either comparable with or shorter than instrument response time.
“When we initially started to use the spectrometer, we were able to generate a reasonable continuum in two ranges: 450 to 750 nm and 850 to 1200 nm using the same sapphire crystal,” Frank said. With the new material for enhanced continuum generation, they now can cover 450 to 750 nm and 950 to 1570 nm in ideal conditions. He said that this is useful especially for wavelengths approaching 1500 nm.
With these measurements, the group determined that dimerization of astaxanthin in α-crustacyanin is the primary molecular basis for the bathochromic shift of the S0 → S2 and S1 → Sn transitions and accounts for the major portion of the transitions, while planarization of astaxanthin leads to a longer effective π-electron conjugated chain, S1, and, consequently, a shorter t1 in the protein than that in solution.
The researchers also have used the transient absorption technique on similar substances such as peridinin in several solvents, revealing the different lifetimes of this molecule’s lowest excited singlet state, from 7 ps in the strongly polar solvent trifluoroethanol to 172 ps in the nonpolar solvents cyclohexane and benzene.5
Nanoparticle physics
Scientists expect that nanoparticles will lead to new targeted drug delivery mechanisms, light sources and microchip designs, among other applications — and those are the examples we can see now.
Professor Prashant Kamat of the University of Notre Dame’s department of chemistry and biochemistry is part of a group exploring the novel properties and uses of nanoparticles on the ultrafast scale. His work has focused on photoinduced catalytic reactions using semiconductor and metal nanoparticles, nanostructures and nanocomposites, advanced materials such as inorganic-organic hybrid assemblies for using renewable energy resources, and environmental remediation using advanced oxidation processes and chemical sensors.
Semiconductor and noble metal clusters in the nanometer-size regime display many interesting optical, electronic and chemical properties that are size-dependent. Such materials have potential applications in developing biological sensors and optoelectronic devices. Research activity in recent years has focused on the synthesis and organic functionalization of metal nanoparticles of various shapes and sizes. The size- and shape-dependent optical and electronic properties of semiconductor and metal nanoparticles make an interesting case for photochemists and photobiologists to exploit their role in light-induced chemical reactions.
Binding a photoactive molecule (e.g., pyrene) to a metal nanoparticle enhances the photochemical activity and renders the organic-inorganic hybrid nanoassemblies suitable for light-harvesting and optoelectronic applications. The nature of charge transfer interaction of fluorophore with a gold surface dictates the pathways with which the excited state deactivates. Obtaining insight into energy and electron transfer processes is important for improving the charge separation efficiencies in metal-fluorophore nanoassemblies and the photocatalytic activity of metal-semiconductor composites.
According to Kamat, who used the Helios spectrometer to study the photophysical properties of metal nanoparticles, labs would typically spend up to a year developing the ultrafast spectrometer before making the first measurement.
But a person with minimal knowledge of this technique can start using this system in minutes. “You start the laser, place the sample, and after a little fine tuning, the system starts collecting data very nicely,” he said.
Previously, Kamat used a 1-kHz ultrafast laser, but the homemade spectrometer could collect data only at 200 Hz, or only on every fifth pulse.
Curing DNA damage
Researchers at the Service de Bioénergétique of the Commissariat à l’Energie Atomique in Saclay, France, are studying how absorption of UV light leads to chemical modification of DNA. If not repaired, such lesions can result in cell death, mutations and cancer. DNA photolyase is a flavoprotein that serves in a variety of organisms to repair UV-induced lesions in DNA. DNA repair is initiated by a near-UV or blue photon. This enzyme contains a flavin adenine dinucleotide (FAD) as the essential catalytic cofactor and a second chromophore, whose sole function is absorbing light and transferring excitation energy to the FAD cofactor.6 This cofactor must be in doubly reduced form (FADH-) for the enzyme to repair DNA.
The main type of the UV-induced lesions, a cyclobutan dimmer of neighboring pyrimidines, can be split by light-induced electron transfer from the FAD cofactor of photolyase toward the damaged DNA. In E. coli photolyase, several stages of electron transfer occur. Transient absorption spectroscopy techniques helped the researchers understand these transfer processes during the photoactivation of E. coli photolyase.2
Other transient absorption studies on DNA include triplet states and ionization of oligomers, where the transient spectra obtained for the single and double helices are compared with those of the nucleotides thymidine monophosphate and deoxyadenosine monophosphate.7
Initial experiments using a spectrometer built in-house had a time resolution of 0.5 ms, while Helios offers subpicosecond resolution, significantly increasing a researcher’s ability to collect transient spectra.
With the advent of fast readout electronics and stable optical designs, spectrometers are making it possible for researchers to collect transient absorption spectra as fast as optical probes can produce them. System development, testing, calibration and setup times have been cut from the better part of a year, involving physicists, chemists and biologists, to a few minutes by a trained technician, while expanded continuum probes push farther into the critical infrared portion of the absorption spectrum.
Meet the authors
Jay Jeong is product manager at Newport Corp. in Irvine, Calif.; e-mail: [email protected].
Alexei Gusev is director of R&D at Ultrafast Systems LLC in Sarasota, Fla.; e-mail: [email protected].
References
1. G.V. Hartland (2004). Measurements of the material properties of metal nanoparticles by time-resolved spectroscopy. PHYS CHEM CHEM PHYS, Vol. 6, pp. 5263-5274.
2. M. Byrdin et al (July 22, 2003). Dissection of the triple tryptophan electron transfer chain in Escherichia coli DNA photolyase: Trp382 is the primary donor in photoactivation. PNAS, pp. 8676-8681.
3. D. Frolov et al (2005). Investigation of B-branch electron transfer by femtosecond time-resolved spectroscopy in a Rhodobacter sphaeroides reaction centre that lacks the QA ubiquinone. Biochim et Biophys Acta, 1707, pp. 189-198.
4. C. Aubert et al (May 11, 1999). Intraprotein electron transfer between tyrosine and tryptophan in DNA photolyase from Anacystis nidulans. PNAS, pp. 5423-5427.
5. D. Markovitsi et al (2003). The effect of molecular organisation in DNA oligomers studied by femtosecond fluorescence spectroscopy. Chem Phys Chem, Vol. 4, pp. 303-305.
6. R.P. Ilagan et al (2005). Femtosecond time-resolved absorption spectroscopy of astaxanthin in solution and in αCrustacyanin. J Phys Chem A, Vol. 109, pp. 3120-3127.
7. J.A. Bautista et al (1999). Excited state properties of peridinin: Observation of a solvent dependence of the lowest excited singlet state lifetime and spectral behavior unique among carotenoids. J Phys Chem B, Vol. 103, pp. 8751-8758.