The technique has been around for years and shows great promise, but so far researchers have been frustrated in their attempts
at clinical translation.
Imaging techniques using UV-VIS spectroscopy hold promise for a range of important clinical applications. But despite years of development leading to robust technologies that have undergone thorough testing, commercialized instruments are having a tough time finding their way into the clinic.
BioPhotonics checked in with the developers of two of these techniques to get a sense of why, and to ask what technological challenges remain before the instruments can be implemented in clinical settings. The answer was that there are none. The instruments are ready to go. This, of course, begged the question: What are the obstacles?
Dr. Irving Bigio of Boston University has been developing the technique called elastic scattering spectroscopy (ESS) since the 1990s. ESS provides real-time, semiquantitative, point-source measurements of microstructural changes, and because it is robust and can be miniaturized, it is easily incorporated into endoscopic biopsy tools.

To facilitate clinical adoption of the technology, Bigio’s team has developed elastic scattering spectroscopy devices that easily integrate into the doctor’s work flow. One of the ways they have done this is by incorporating the optical fibers into the biopsy forceps that the doctor uses to remove growths. Courtesy of Irving Bigio, Boston University.
Bigio and colleagues have successfully demonstrated ESS for a range of applications in the upper gastrointestinal (GI) tract and in the colon – determining the presence of dysplasia in Barrett’s esophagus, distinguishing benign from adenomatous polyps in the colon, or helping to differentiate Crohn’s disease from ulcerative colitis – while also showing its potential for detecting cancer in thyroid nodules and for real-time pathology to guide breast surgery.
In the June issue of Inflammatory Bowel Diseases, Bigio’s team reported a study in which they demonstrated the use of ESS as an optical marker of inflammatory bowel disease activity, showing its potential for discriminating between ulcerative colitis and Crohn’s disease.
As part of the study, they devised a diagnostic algorithm based on a pattern recognition scheme to classify measured spectra. The algorithm allowed them to determine whether a given diagnosis from an optical biopsy has a high confidence of being correct. This increases the reliability of those measurements where the instrument does make a call. And for the 10 to 15 percent of cases where the algorithm indicates that there’s still some uncertainty, the doctor can take additional measurements or perform a physical biopsy, as per standard clinical practice.
The technology is mature and could clearly benefit a range of clinical applications. But it’s still a few steps away from actually entering the clinic. Two significant obstacles have gotten in the way of this. The first is a familiar one: reticence on the part of clinicians, who are often wary of adopting new technologies, especially those that might have a steep learning curve. To address this, Bigio and colleagues have been working to design devices that seamlessly integrate into the clinician’s work flow – for example, by incorporating the optical fibers into the biopsy forceps used to remove polyps during endoscopies. They also have worked to do so in a way that is manufacturable. Clinicians have tested this new design, he said, and have been very enthusiastic about it.
But there’s still another – likely more intractable – obstacle: attracting funding from an investment community that is increasingly averse to any kind of risk.
Bigio’s group has performed large-scale clinical studies with elastic scattering spectroscopy and has met the performance standards established by the American Gastroenterological Association for an optical biopsy tool for colonoscopy or upper GI endoscopy: 90 percent sensitivity and 90 percent negative predictive value. They are confident that the technology is ready for prime time. But still investors are reluctant to embrace it.
“There’s no venture in venture capital anymore,” Bigio said. Groups will say they are interested in a new technology, but only after it has received FDA approval. They don’t want to get involved in funding the FDA trials.
This has become a problem across the medical instrumentation field. The trend these days is that when larger companies want new products, they simply buy existing companies. Bigio offers an example of this: Covidien bought Given Imaging, which had developed the PillCam capsule endoscopy system. And then Medtronic announced plans to buy Covidien.
But this sort of development strategy is shortsighted, he said. Companies will buy other, smaller companies for $800 million or $900 million, “but they won’t spend $5 million to fund a new idea that will open a whole new product area.”

Researchers are working to commercialize spectroscopy-based technologies for the clinic. Dr. Adam Wax of Duke University formed the company Oncoscope to commercialize angle-resolved low-coherence interferometry technology and is working toward FDA approval. Courtesy of Adam Wax.
Bigio’s team isn’t the only one to encounter this difficulty in attracting funding. Dr. Adam Wax’s group at Duke
University has been developing a technique – angle-resolved low-coherence interferometry, or a/LCI – enabling early detection of cancer and other biomedical applications by measuring the average size of cell nuclei using scattered light.
The focus of the a/LCI instrument has been Barrett’s esophagus, a precursor to esophageal cancer. But Wax is also looking beyond the upper GI tract. In his research lab at Duke, he and colleagues have been working to develop the technology further, to bring it to bear on cervical cancer, which has different requirements than applications in the esophagus. They have been building an instrument that is designed for cervix studies with a Duke clinical team and have recently delivered it to partners at the University of California, San Francisco, where they have access to a cohort of patients that will enable a thorough testing of the device.
Wax and colleagues formed the company Oncoscope in 2006 to explore commercialization of a/LCI technology and have recently revamped the technology to make it more suitable for clinical use. The new instrument is designed for a better fit in the endoscopy suite, the software is more intuitive, and they are developing a swappable probe and a disposable sheath. But the company has run up against the same reluctance in the investment community.
“A number of people have told us, ‘If you get [FDA] approval, we’ll finance you,’ ” Wax said. But few were willing to get involved in the early stages of development. They are now working with a corporate investor – Applied Ventures, the venture arm of Applied Materials – that is helping them navigate the process; however, as it has been for many others looking to introduce new technologies into the clinic, the road has proved a long one.
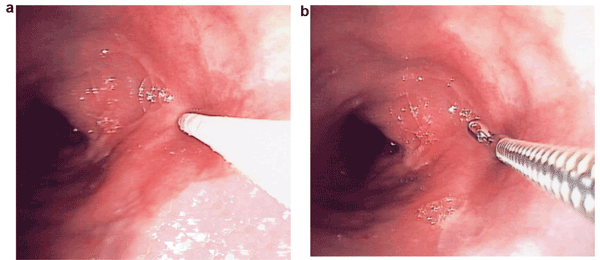
Researchers at Boston University are developing a range of UV-VIS spectroscopy-based technologies to aid in clinical applications – for endoscopic assessment of esophageal and colon cancers, for example. Optical ‘biopsies’ (b) offer noninvasive assessment and an instantaneous response. This means doctors can make a determination about a growth without having to cut it off and retrieve it, as they would with surgical biopsies (a). Courtesy of Irving Bigio, Boston University.
Alternative path to commercialization
Bigio, Wax and the other developers of spectroscopy-based technologies continue to work to obtain funding to commercialize their instruments. It is important that they do this, as getting the instruments into the clinic would truly benefit endoscopic and other applications. But they are also looking at other means to apply UV-VIS spectroscopy in the biomedical arena. At Duke, for example, Wax and colleagues have been developing cellular assays in a lab-on-a-chip format − using UV spectroscopy to analyze functionalized gold nanoparticles, for example. This is “a good way to get biophotonics technologies into the market without requiring the same FDA regulatory hurdles as with Oncoscope,” he said.
Funded by a grant from the National Science Foundation, the researchers have developed a technology with which to perform holographic imaging of cancer cells inside microfluidics, adding molecular specificity – which isn’t available with a/LCI – by using the gold nanoparticles. Tests with the technology have already yielded very exciting results, providing new biophysical insights and diagnostic capabilities that Wax and colleagues are preparing to publish within the next several months.