Researchers in the mechanical engineering department at Toyohashi University of Technology (TUT) have developed a low-cost photoporation method using titanium-oxide nanotubes. Photoporation is a technique that relies on focused light to perforate the cell membrane, creating an opening through which substances may enter the membrane.
Because photoporation has in application traditionally used gold nanoparticles, which absorb pulsed light, the technique has often been expensive to practice. The technique developed at TUT, however, uses an alternative, more cost-effective material: titanium-oxide nanotubes (TNTs).
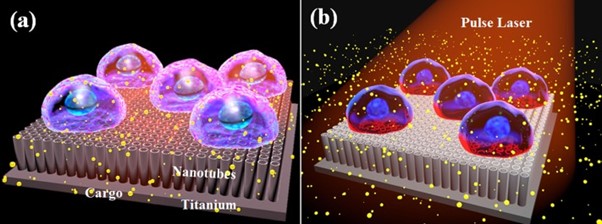
Representation of (a) cells cultured on top of titanium-oxide nanotubes and (b) massively parallel photoporation using the interaction between an array of nanotubes and a pulse laser. Courtesy of Toyohashi University of Technology.
The TNTs were formed on titanium sheets at different voltages and times using electrochemical anodization. X-ray photoelectron spectroscopy showed different types of titanium oxide, such as TiO2 and TixOy (TiO/Ti2O3/Ti3O5). Different anodization voltages and times yielded different concentrations of these variants, as well as a minor quantity of Ti metal. Due to the formation of oxygen defects, nanotubes have quasi-metallic and metallic properties.
Upon irradiation with a nanosecond pulse laser, these properties may facilitate the intracellular delivery through various mechanisms after irradiation with a nanosecond pulse laser.

The researchers cultured human cervical cancer cells on TNTs and introduced a biomolecular solution. After exposure to a 532-nm laser pulse, the researchers delivered propidium iodide and dextran into the cells with high degrees of efficiency and cell viability.
“The precise mechanism for the intracellular delivery on TNT-based photoporation is still unclear,” said L. Mohan, a researcher at TUT.
Among the possible principles of cell membrane perforation are thermal-mediated nanobubbles; photochemical-induced reactive oxygen species (ROS); heat transfer from nanotubes to the cell membrane; and localized surface plasmon resonance (SRS) high electromagnetic field enhancement on each nanotube. Cavitational nanobubbles form in the cell membrane-nanotube interface and can rapidly grow, coalesce, and collapse, causing explosions that result in the perforation of the membrane, allowing biomolecules to enter the cell.
“Intracellular delivery may happen by the combination of the mechanisms,” Mohan said.
According to team leader Moeto Nagai, titanium-oxide nanotubes could be a versatile and low-cost platform for intracellular delivery with laser pulses, as the technology achieves parallel and controlled uniform delivery with high efficiency and cell viability. The technique could eventually see use in cellular therapy and regenerative medicine.
The research was published in Applied Surface Science (www.doi.org/10.1016/j.apsusc.2020.148815).