Solid-state green light-emitting devices have experienced a growth spurt in recent years, greatly increasing their availability and opening doors to new applications.
FAIZ RAHMAN, OHIO UNIVERSITY
Human eyes are most sensitive to light in the yellow-green region of the visible spectrum. This well-known fact is a result of the way mammalian eyes, and for that matter the eyes of all land-dwelling animals, have evolved from those of aquatic creatures. Electromagnetic radiation falling in the visible spectrum passes relatively unattenuated through water, and animal eyes have evolved to take advantage of this situation.

Our eyes see light spanning roughly the wavelength region from 400 to 700 nm, with most sensitivity appearing at the center of this interval. Yellowish-green color is where our eyes’ sensitivity peaks (Figure 1). This has important implications when it comes to developing artificial light sources for illuminating our living and working environments.
Any decent light source should have a relative abundance of wavelengths in the central part of the visible spectrum. These and other considerations have led scientists and engineers working on solid-state light sources to pay particular attention to developing green light-emitting devices. Interestingly, however, whereas emitters in the red and blue-violet regions have been relatively easy to develop, green emitters have presented considerable challenges. Until recently, this resulted in a conspicuous shortage of affordable and efficient solid-state green light emitters. This state of affairs continued for many years, well into the 21st century, and came to be known as the “green gap.” However, with the persistent efforts of many researchers and technologists, the green gap has shrunk almost to the point of nonexistence, and light-emitting devices are now available in this color region.
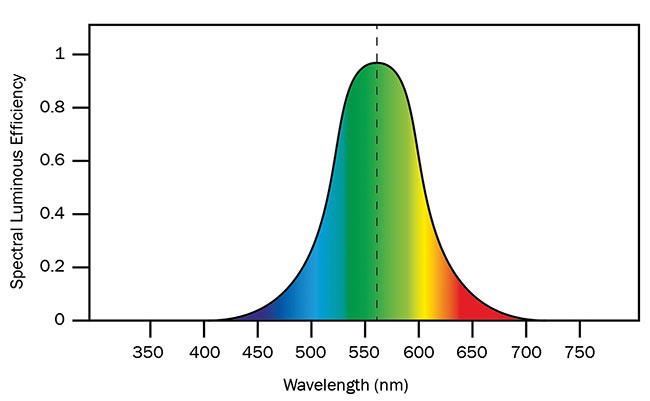
Figure 1. Photopic response curve for human vision normalized to maximum response at 555-nm wavelength. Courtesy of National Physical Laboratory.
Because of the inability of silicon to emit light of any kind efficiently, all light-emitting devices — whether light-emitting diodes (LEDs) or laser diodes —
are made from compound semiconductors. III-V semiconductors such as gallium nitride (GaN) and gallium arsenide (GaAs) are widely used for this purpose. LEDs that emit green light have been made, for a long time, using the gallium phosphide (GaP) material system. Usually, the substrate is a GaP wafer on which various other semiconductor alloy layers are grown through metalorganic chemical vapor deposition (MOCVD) process. The active pn-junction and quantum well layers are made from aluminum gallium phosphide (AlGaP) and aluminum indium gallium phosphide (AlInGaP) alloys — all grown epitaxially on top of the GaP wafer.
Recent advancements
Advancements in both semiconductor processing and device packaging have been behind some notable advancements in optical power output of green-emitting LEDs. MOCVD growth and related processing of green LED wafers made from AlGaP-based heterostructures have matured over the past two decades so that the operating efficiency has nearly doubled. This has led to both increased output power and enhanced emission efficacy.
Concomitantly, there have been developments in new, low thermal-resistance packaging that have enabled placing either a large die or multiple smaller dies on the same physically small chip carrier. Both chip-on-board (COB) and multiple-chip-on-board (MCOB) packages have been developed that feature low thermal resistance from the back side of the LED chip to the bottom of the package. State-of-the-art commercial packages have thermal resistance as low as 0.64 °C/W. These are now the standard for all green LEDs that emit from 100 to 1000 lm of optical power.
For even higher output, ceramic and sintered metal powder packages have been developed that allow placement of several LED dies in close proximity. Having multiple chips abutting each other and wired in parallel drives up the power capability to several thousand lumens per package. Such “light engines” are currently the ultimate in brightness, as far as solid-state, noncoherent green light sources are concerned.
Intrinsic green LEDs
Green LEDs fall into two major categories: those made with semiconductors that are intrinsically capable of emitting green light, and those that are based on a blue-emitting LED chip topped with a green conversion phosphor.
Intrinsic green LEDs derive their color from the bandgap of the semiconductor alloys used in the construction of the pn-junction, as well as the exact thicknesses of the quantum well and barrier layers that form the light-emitting region. The alloy composition is chosen so the bandgap of the alloy lies close to the desired emission wavelength. The bandgap is the difference in energy between the lower end of the conduction band and the upper end of the valence band. It controls the energy (and thus the wavelength) of the photon that is emitted as an electron recombines with a hole. Further wavelength tuning is affected by adjusting the precise thicknesses of the well and barrier layers that form the quantum well region at the center of the LED layer structure.
Most commercial LEDs are based on a standard structure with five quantum wells, as this offers the best compromise between efficiency (light output) and growth complexity (cost). With a given alloy composition, making the multiple quantum well (MQW) layers thinner reduces the wavelength (blue shift) of the emitted light, whereas making the wells thicker has the opposite effect. Together, alloy composition and MQW layer thicknesses determine the exact peak emission wavelength.
The width of the emission, however, is governed, among other factors, by the disorder in the alloy (spatial composition fluctuations) and variations in MQW layer thicknesses. Limits on semiconductor alloy stoichiometry — arising out of miscibility and phase separation considerations, and various physical effects such as carrier leakage and nonradiative recombination — govern the ultimate achievable efficiency of LEDs.
Thus, even with state-of-the art growth and processing, there is only so much electrical-to-optical conversion efficiency that can be attained by these devices. Present-day semiconductor materials and manufacturing technology may have reached near full attainable efficiency. Any future increases in this metric would therefore be incremental at best. At the time of this writing, a number of manufacturers are offering bright green bandgap emission LEDs in relatively small form factors (Figure 2).
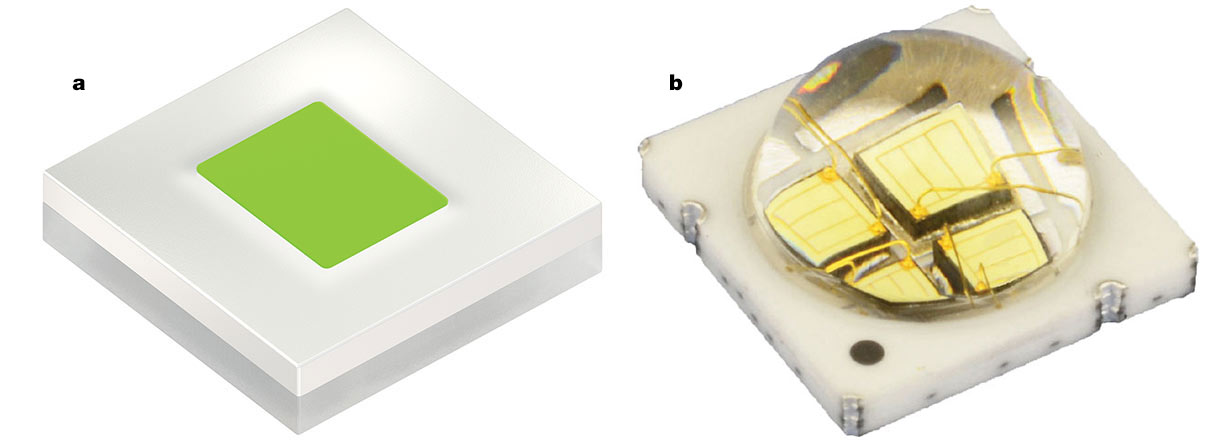
Figure 2. Typical commercial high-brightness bandgap emission green LEDs. This illustration shows devices from Osram (KP CSLPM1.F1 — 680 lm) (a) and LED ENGIN (LZ4-00G108 — 657 lm) (b). Courtesy of Osram Optosemiconductors.
Phospor-converted LEDs
The other major type of commercial green LEDs are devices that unite a blue-emitting GaN/InGaN (indium gallium nitride) LED chip with a wavelength-converting phosphor. Such devices benefit from the rapid advancements that have been made in blue LEDs, the techology of which lies at the heart of all so-called white LEDs. Market demand for illumination-quality white LEDs has seen to it that blue LEDs that pump phosphors to generate white light have nearly reached a pinnacle of perfection.
The same approach could also be used to generate green light. This scheme makes use of blue-power LED chips to energize an appropriate down-conversion phosphor, which shifts the wavelength from blue to green. (Note that “down-conversion” here refers to the energy or frequency of the photons going down, whereas the wavelength increases when one converts from blue light to green light.) While this strategy benefits from the availability of efficient blue LEDs, it suffers from conversion losses in the phosphor.
Market demand for illumination-quality white LEDs has seen to it that blue LEDs that pump phosphors to generate white light have nearly reached a pinnacle of perfection.
Unlike the case with phosphor-converted white LEDs, green LEDs that employ a blue-emitting chip pumping a phosphor need to ensure that all blue light is consumed by the phosphor so there is no contamination of the down-converted green light from residual unconverted blue light. In contrast, white LEDs make use of purposely bled residual blue light, together with phosphor-generated yellow light, to create their white appearance. This requirement puts stringent demands on both the conversion efficiency and the coating thickness of the green-conversion phosphor.
Only phosphors with high quantum efficiency will work for green phosphor-converted LEDs, and a substantial amount of this phosphor has to be used to make sure that all pump light is converted to green light. Sources of phosphor-related efficiency loss include less than perfect quantum efficiency, scattering loss among phosphor particles, and Stokes shift. The last refers to the energy that is lost as blue photons are converted to lower-energy green photons.
An additional loss mechanism with phosphor-conversion LEDs arises because of the thermal blanketing effect of phosphor coatings on LED chips. By restricting radiative heat loss from the top surface of the pump chip, the presence of a phosphor coating increases the temperature of the LED chip during operation. This results in a small loss of efficiency and also limits the long-term service life of the phosphor through thermal degradation. Careful material selection and integration, however, have resulted in the development of several lines of commercially successful phosphor-converted green LEDs.
Traditional phosphors, which are generally based on rare-earth ions acting as the light-conversion centers hosted in a dielectric inorganic crystal, have been the mainstays of both white and green LEDs based on phosphor conversion1. Several green-emitting phosphors are commercially available for this purpose from companies such as Materion and Intematix. Examples include: K3Gd(PO4)2:Tb, NaCaPO4:Eu, Ba2Gd2Si4O13:Eu, and Lu3Al5O12:Ce. Many others have been reported in the literature.
The green emission arises from crystal field-determined transitions between the energy levels of the rare-earth dopant. The exact emission wavelength is dependent on both the composition of the host crystal and the identity of the rare-earth atom with which it is doped. Typical wavelength down-conversion efficiencies range from 50 to 80 percent.
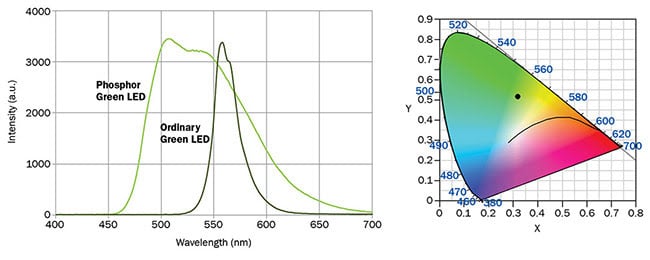
Figure 3. Spectrum of light emission from Electrospell ESL2G11 series LEDs. The graph (right) shows the chromaticity point for this LED. Courtesy of Electrospell.
Phosphor-conversion green LEDs are easy to distinguish from bandgap emission green LEDs because they usually have a very wide spectral output. The price of large spectral bandwidth, how-
ever, is paid through loss of color saturation, which shows in the chromaticity point of such an LED (Figure 3). The remote location of the chromaticity point from the diagram’s boundary implies that the light is highly nonmonochromatic and it appears as a pleasant apple-green color. As mentioned below, this feature is actually desirable for constructing broadband RGB light sources.
Quantum dot LEDs
It is also possible to construct green-emitting LEDs by combining blue pump chips with other luminescent materials, such as quantum dots. These are essentially nanocrystals of materials, such as cadmium selenide (CdSe), that range in size from ~2 to 20 nm in diameter. Because of their small size, quantum dots show strong quantum confinement effects and are akin to artificial atoms, as far as their energy levels are concerned. LEDs based on quantum dots have been fabricated by a number of research groups2. While these devices have been demonstrated several times in the past, they have yet to be commercialized.
Quantum dot LEDs emit light with much narrower spectral spread than phosphor-conversion LEDs, and this can benefit certain applications such as screen- and projection-based displays. In this attribute, quantum dot LEDs are opposite of phosphor-based LEDs. The cost of good-quality quantum dots and the issue of their longevity have so far precluded their use in commercial green LEDs. While yet to make an appearance in commercial discrete LEDs, quantum dots have found an application in embedded LEDs in televisions from companies such as Samsung Electronics of Suwon, South Korea.
Laser diodes
While green-emitting LEDs have been in existence for decades and have rapidly improved in recent years, commercial laser diodes that emit green light are a recent development. Laser diodes, as a general rule, are much harder to make than LEDs. Their material structure and epitaxy are both far more challenging than is the case with LEDs. They also require a level of perfection in substrate and material growth that significantly exceeds similar requirements encountered with LED fabrication.
Laser diode layers have to be grown on extremely well-matched metamorphic substrates. Otherwise, lattice mismatch leads to extensive threading dislocations that cause nonradiative electron-hole recombinations, thus killing laser action. Even the potential of dislocation formation is bad news because as devices age, dislocations can multiply and turn into dark-line defects (DLDs) that diminish the power output of lasers and eventually stop the lasing action altogether. Given these considerations, it is no wonder that making laser diodes is quite complicated and these devices cost substantially more per unit than LEDs.
Red laser diodes were the first visible-color semiconductor diode lasers that were developed, and their technology has matured so much that they now cost very little and are widely used in many applications that require a small source of directional light beam. Blue laser diodes were developed next, using the GaN/InGaN material system, and their technology has also been well-developed since.
Green laser diodes have been approached using the same GaN/InGaN material system but with significantly higher indium concentration in the InGaN ternary alloy. Increased indium content narrows down the bandgap and thus shifts the emission wavelength from blue toward the green region3
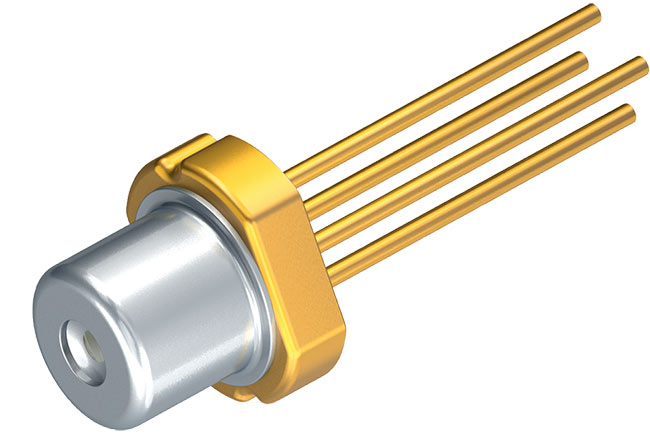
Figure 4. Osram green laser diode PL 520. This device emits 30 mW of optical power at 515 nm. Courtesy of Osram Optosemiconductors.
Challenges with increasing the amount of indium in the InGaN alloy to values that would allow green emission have held back the development of green laser diodes for many years. At high indium content, phase separation leading to alloy stoichiometry fluctuations rapidly becomes severe, and obtaining spatially homogenous material becomes increasingly difficult. Intense research over the past decade has succeeded in developing growth techniques that allow InGaN alloy with high indium content to be grown without excessive phase separation4. This, in turn, has allowed the manufacture of commercial green laser diodes at acceptable per unit cost (Figure 4). However, their fabrication technology is quite immature and the yields are relatively low. Thus, green laser diodes are still somewhat expensive and not as widely available as their red and blue variants.
It is also possible to combine blue-violet laser diodes with a suitable green-emitting phosphor to make bright, narrow beam, incoherent green light sources. This approach is now already used with white light sources for automotive headlamp applications in high-end automobiles5. Such incoherent green sources can generate concentrated light for signage and projection systems.
Increasing applications
The availability of bright green solid-state sources is enabling several new applications. Owing to our eyes’ superior sensitivity in the green region, green LEDs are very suitable for monochrome displays that are visible from long distances. Large public announcement display boards and over-the-air visual signaling systems operating over several miles can make use of widely available low-cost green LEDs, light engines, and multi-LED modules.
Green LEDs can also be used along with red and blue LEDs for making RGB LED tunable white light systems that allow the color of mixed light to be tuned at will across a broad color space. There are two distinct possibilities here: 1) For RGB LED systems that provide back illumination for LCDs; narrowband red, green, and blue LEDs made from band-edge emission semiconductors are suitable; and 2) for LED-based projection systems; several of Luminus Devices’ RGB chipsets are geared toward this application.
For reflected light illumination of real physical objects and living spaces, it is best to combine a phosphor-converted green LED with red and blue LEDs, either narrowband or broadband. This is because a reflected light application demands spectral richness from the source of illumination in order to correctly render the color of objects seen under reflected light.
While green-emitting LEDs have been in existence for decades and have been rapidly improved in recent years, commercial laser diodes that emit green light are a recent development.
Green phosphor-conversion LEDs can have very wide spectral output centered in the green region (Figure 3). This feature works extremely well in conjunction with light from red and blue LEDs to synthesize light with broad (and relatively flat) spectral coverage. Even white LEDs have benefitted from the development of
phosphor-converted green LEDs. The special broadband green phosphors developed for the latter have also been used in white LEDs to make flat-spectrum broadband LEDs, such as the Flat-white LED from Electrospell.
Green laser diodes
Green laser diodes open the doors for several other applications. First of all,
they are providing a more compact and reliable replacement for many applications that have hitherto been served by traditional diode-pumped solid-state (DPSS) green lasers. As green diode lasers gain in power over the coming years, more applications will migrate from the use of DPSS green lasers to pure diode lasers. Laser-based illumination, just like LED-based illumination, stands to gain here as well. These lasers can be combined with red and blue diode lasers for laser projection displays, and this has been a major incentive for their development.
Both LCD-based and micromirror-based projection devices benefit from this development.
Green diode lasers also offer the possibility of direct high-speed electronic modulation — a capability that is not offered by their DPSS counterparts. This can directly benefit both visual-band, over-ground and ground-to-space optical communication systems. In fact, several experiments with microsatellites are now exploring both ground-to-space and space-to-space communications links with green laser diodes.
Yet another application possibility is the use of green laser diodes for underwater communications systems. Although water’s transmission exhibits its highest peak at 418 nm, green wavelengths are still close enough to be of almost equal value for underwater communication links. In fact, in most real aquatic environments, water shows the least absorption approx-
imately halfway between the blue and green regions, and thus green lasers are very suitable to serve in optical communications links in such environments.
The above possibilities are only a handful of the vast application spectrum that is and will be enabled by bright, affordable, diode-based green light sources. Green LEDs, especially bandgap emission LEDs, will further grow in brightness in the coming years. Green-emitting laser diodes, however, require much more development so that their price comes down further, together with an increase in their availability. Hopefully, one day direct diode-based green lasers will replace almost all DPSS green lasers.
This survey has shown that the green gap has diminished greatly from earlier years, with a variety of devices now available on the market. More R&D is still needed for green laser diodes, but with their commercialization, a milestone has been achieved and we can look more confidently toward the future.
Meet the author
Faiz Rahman, Ph.D., is a faculty member at Ohio University. His interests include electronic and photonic materials and devices. He is a senior member of the Optical Society
of America and also of the IEEE; email: [email protected].
References
1. F. Rahman and W. Jadwisiencsak. (2014). Phosphors: the driving force behind white LEDs. Compd Semicond, Vol. 20, Issue 56.
2. Y. Araki et al. (2007). Green light emitting diodes with CdSe quantum dots. J Crys Growth, Vol. 809, pp. 301-302.
3. S. Lutgen et al. (2010). Progress of blue and green InGaN laser diodes. Proc SPIE, Vol. 7616, 76160G.
4. D. Sizov et al. (2012). Gallium indium nitride-based green lasers, J Lightwave Technol, Vol. 30, Issue 1.
5. A.F. George et al. (2016). Laser-driven phosphor-converted white light source for solid-state illumination, Appl Opt, Vol. 55, p. 1899.