Using the wavelength range applied to materials analysis could differentiate changing water content and blood flow in the body, indicating cancer, diabetes, and burn wounds, though technical challenges remain in system design.
Terahertz imaging has long been used in manufacturing
applications, such as materials and pharmaceutical quality inspection, or in illicit drug and explosives detection.
Due to its low photon energy and ability to penetrate certain types of materials, terahertz imaging holds a great deal of promise for the identification of key biological indicators — particularly those inherent in the skin — in life sciences and medicine. Many molecular processes and structural changes are, in fact, recognizable in this band, but exploiting this fact has proved to be difficult to commercialize. And yet, researchers are getting closer to devising real-world medical applications for terahertz systems that could be employed in clinical settings beyond the laboratory.
The terahertz wavelength range occurs between 30 µm and 3 mm on the spectrum, between the microwave and infrared, with a frequency range of 0.1 to 10 THz.
Tissue hydration has been the focus of considerable research using terahertz imaging. Courtesy of iStock.com/wangmando.
Terahertz imaging has long been used in manufacturing applications, such as materials and pharmaceutical quality inspection, or in illicit drug and explosives detection, because of its ability to probe through materials, such as semiconductors, wood, and polymers. Unfortunately, many biomolecules are too small for individual identification within the terahertz band. Therefore, terahertz imaging is often used in conjunction with near-field imaging and denoising algorithms to capture the nonlinear response of the terahertz wave. Nonlinear optical effects facilitate pulse compression, which can produce high resolution at the molecular level.
For clinical use of terahertz technology, an additional complication is that terahertz waves are highly absorbed in water and therefore do not travel deeply into most tissue. This characteristic is considered a major stumbling block to using terahertz waves on in vivo specimens, which hold a high degree of water content. Others, however, have come to see this absorption as an advantage, because hydration of tissue is often indicative of its health (opening image).
View of terahertz expands
And while companies, such as TeraSense, generally implement terahertz imaging technology in the industrial space, the company points to promising research in areas such as noninvasive cancer detection, because water and certain protein content is higher in tumors. Studies using time-domain terahertz spectroscopy, in which the company has not directly been involved, show that the technique can differentiate healthy skin tissue from basal cell carcinoma, which is the most common form of skin cancer and typically grows slowly. The technique’s inherent ability to capture both frequency and time-domain data (meaning not only the existence of a signal but how it changes over time) could also potentially enhance biopsies of breast and muscle tissue.
In the future, research in the terahertz regime could translate into clinical applications in dentistry, diagnosing the health and thickness of enamel and dentine, and without the potentially harmful radiation that is a byproduct of x-rays traditionally used for this purpose.
Terahertz images identified hydration thresholds that showed the potential risk
of ulceration.
Dmitriy Romanyuk, a sales and marketing manager for TeraSense, said that TeraSense has not employed terahertz systems for this purpose yet, but the number of TeraSense matrix and linear terahertz imaging scanner systems sold to various medical research organizations is increasing. The company’s cameras use semiconductor detector arrays with a spectral range from 50 GHz to 0.7 THz and provide a pulse rate and acquisition speed suitable for many conveyor applications as well as nondestructive security screening.
“In the terahertz range, certain types of materials are known to be transparent, so our customers use our systems to inspect plastic, paper, rubber, and cotton, for example,” he said. “And this imaging can be done in real time, which helps detect defects in materials like ceramics and crystals.”
In certain applications, an object can be dragged across a sensor with images taken at a speed of up to 5000 fps. These sensors — with various pixel sizes, such as 1.5 × 1.5 mm; 0.5 × 0.5 mm; and 1.5 × 3 mm, etc. — have capabilities in both transmission and reflection modes. Transmission mode describes when the wave passing through a sample is measured, and reflection mode covers when the reflected wave that bounces back from a material or tissue surface is examined. The former is nondestructive but is also impeded by the water content in the material (or presumably tissue).
“Transmission mode is what’s used most often when you’re inspecting materials, which must be dry,” Romanyuk said. “But for in vivo diagnostics, such as skin or blood analysis, you could use this technology in reflection mode, because the body has a high degree of water content. Terahertz spectroscopy is already used extensively in the pharmaceutical industry, but its use in medical diagnostics is underdeveloped.”
A burning image
While many biomedical and life sciences applications of the terahertz regime remain theoretical, researchers have found a concrete purpose for these waves, including M. Hassan Arbab, an assistant professor of biomedical engineering at Stony Brook University. In research sanctioned by the U.S. Army Medical Research, Arbab and his colleagues have been using terahertz imaging to assess the severity of burn wounds — potentially helping medics in the field determine which skin tissue will heal, and which needs timely intervention, such as excision and grafting (Figure 1).
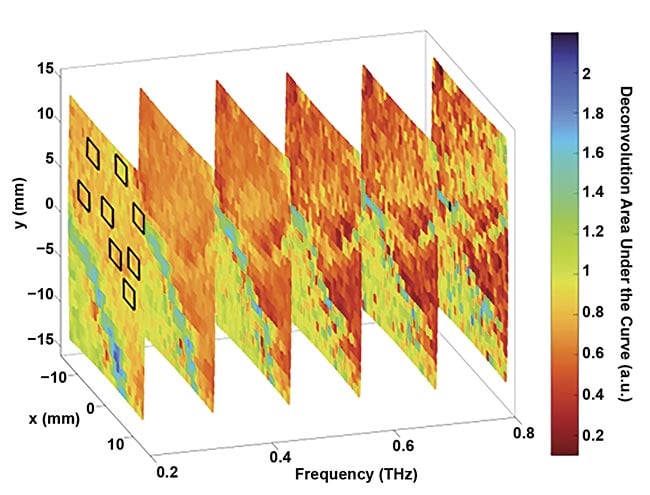
Figure 1. The deconvolution of characteristics captured in tissue in the terahertz range. Courtesy of Hassan Arbab/Stony Brook University.
In their published research a few years ago, Arbab and a team of researchers described the difficulty clinicians can have in differentiating between different types of partial-thickness burns, which damage the epidermis layer of the skin as well as part of the dermis1. Full-thickness burns, on the other hand, affect the entire depth of the dermis, and require surgery. But in certain instances, partial-thickness burns heal without surgical intervention, and in other cases, they expand into other layers of the skin. This progression is dependent on factors such as inflammation, perfusion, and reactive oxygen species, which are unstable molecules that contain oxygen and interact with other molecules in a cell.
“We did our initial studies on pig skin, but in the last one-and-a-half years, we have moved on to human study,” Arbab said. “We receive the doctor’s diagnosis before performing our own imaging scan. In some cases, if the doctor decides to do a skin grafting operation, we will obtain biopsies from the excised tissue for histological staining as the control experiment. In other cases, if skin grafting is deemed unnecessary, we will determine the ground truth by degree of healing up to a month after the injury.”
Traditionally, doctors have waited a few days to determine which category a burn falls into, but this delay can be costly, in terms of the extent of treatment needed and the speed of healing.
So, the Stony Brook team constructed a hand-held, fiber-coupled 2D terahertz time-domain spectral imaging scanner, which they initially used to examine burns in porcine (pig) skin, with measurements of spectral reflectivity of the burn wound over several days. The result was what they called a PHASR (portable hand-held spectral reflection) scanner. The broadband PHASR scanner provides the abilities to measure both the spectral area and spectral slope from each burn. They determined that differentiation in burn wound depth (those involving 50% or more of the dermis) was statistically significant in the data they gathered1.
“The terahertz reading is affected by the density of structures, not only water, and that was part of our analysis,” Arbab said. “There are two sources of epithelial cells: a layer in between the dermis and epidermis, and in compartments around structures, such as hair follicles. The presence of those structures is important for healing to occur.”
More recently, they focused their work on fine-tuning such measurements and creating a model by which other researchers and clinicians can make their own determinations of burn severity. To accomplish this, they focused on the polarization inherent in water molecules, and the contrast present in burns of varying severity (the extent of burn damage in the dermis). Based on several parameters obtained from the Debye dielectric relaxation theory — the process where polarized water molecules reorient themselves in response to an electric field — the group informed an artificial neural network classification of burn injuries. They were able to predict re-epithelialization (the establishment of a protective layer of skin cells) with this model over a period of 28 days2.
To build their device, the Stony Brook team has worked with companies such as Menlo Systems and TOPTICA Photonics. And Arbab said they have also begun researching the value of terahertz imaging in examining the hydration that has occurred in the cornea (i.e., for the diagnosis of dry-eye disease).
“Whereas skin is heterogenous with a lot of scattering, corneal imaging has its own challenges because you’re imaging a spherical structure, but you can account for a rise in intraocular pressure with a change in the terahertz reading,” Arbab said. “For a long time, the high absorption of water in the terahertz range was considered a negative thing. But research like ours shows that this feature can be used as its own contrast mechanism.”
Arbab estimated that the commercialization of this work may take several more years, given the time frame needed for clinical study and regulatory approvals from the FDA and other entities.
Instrumentation advancements
Recent innovations in terahertz system design are making biomedical applications more realistic in concept, even if many of them are not yet clinically available. Anselm Deninger, director of technical sales for TOPTICA Photonics, said that not that long ago, terahertz instrumentation was fit on a board on an optical table, which made it impractical for in vivo use. Now, equipment can be far more versatile, potentially moving to the bedside.
“The systems we use are fiber-based with a photoconductive antenna, and can operate in a safe range for samples,” he said. The company’s TeraScan systems employ diode lasers for excitation with gallium arsenide and indium gallium arsenide (InGaAs) semiconductor photomixer technology, he said.
Deninger said that TOPTICA unveiled the first continuous-wave terahertz laser system at Laser Munich in 2007. The company’s offerings have since expanded to include this continuous-wave terahertz capability in the TeraScan system, but also fiber-coupled, pulsed high-speed and high-bandwidth TeraFlash systems, with InGaAs photoconductive switches.
While the vast majority of their customer base in the terahertz range is still in academic research, he said that industrial users have employed terahertz imaging devices in the manufacturing sector to probe materials characteristics and gauge their thickness (Figure 2). They operate in both transmission and reflection modes, generating amplitude and phase images, and a sophisticated algorithm analyzes the data.
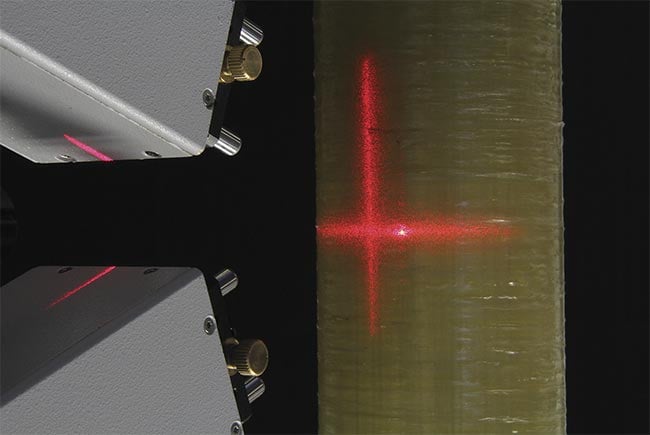
Figure 2. Lasers are used to gauge materials thickness in manufacturing settings. Courtesy of Fraunhoffer ITWM.
“These layers that are being inspected are not homogeneous,” he said. “The inspection of biomolecules can be somewhat similar, in that molecular binding creates the effect to be captured. Even if we’re not directly involved in the work, there are several research projects ongoing to use terahertz in biomedicine, including Hassan Arbab at Stony Brook University [described above] and Enrique Castro Camus, a professor of optics and leader of the Applied Terahertz Science Group at the Centro de Investigaciones en Óptica in Mexico, who has tested the ability to use terahertz imaging to diagnose diabetic foot ulcers. Their work is exciting, and the terahertz market may very well expand because of it.”
Terahertz and diabetes
Camus said that prior to his research into diabetic ulcers, he experienced the same challenges that many others did regarding the strong absorption of terahertz waves in water. But he explained that his study of plant hydration in the early 2010s naturally led into dehydration in human subjects, particularly since diabetic ulcers are common in Mexico and other places around the world.
Diabetic peripheral neuropathy, which causes numbness, tingling, or pain, is felt in the arms and legs and can be a precursor to the onset of ulcers. Standard diagnostic tests include the Semmes-Weinstein Monofilament test, in which a flexible strip is used to touch a patient’s skin to gauge if pressure is felt; and the ankle-brachial index, which compares blood flow in the arm and ankle that would indicate the presence of peripheral artery disease.
Recent innovations in terahertz system design are making biomedical applications more realistic in concept, even if many of them are not yet clinically available.
Camus credited one of his Ph.D. students, Goretti Hernandez, with securing some of the early measurements, and a fortuitous partnership developed with Blanca Murillo-Ortiz, a full-time researcher at the High Specialty Medical Unit of IMSS-Leon.
In the team’s published research, they described their technique, called moisture mapping by terahertz (MMAT), which captures the variation in hydration in the soles of the feet (Figure 3). Terahertz images identified hydration thresholds that showed the potential risk of ulceration. To obtain their images, they used a TeraGauge spectrometer from Advanced Photonix Inc. Femtosecond pulses were sent through an ytterbium fiber laser and split between a photoconductive transmitter and a receiver3.
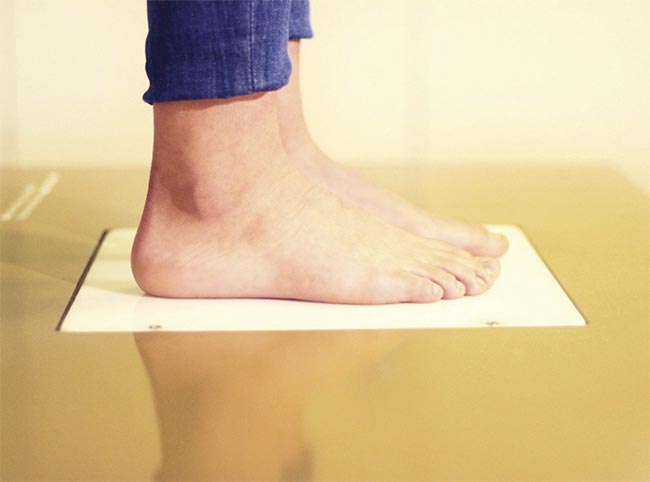
Figure 3. Feet are photographed on the scanner window. Courtesy of E. Castro-Camus and P. Almendarez.
Based on the age and health of the patients they examined, they determined that all patients lose hydration as they get older, but this process happens more quickly and severely for those who suffer from diabetes (Figure 4). Just as importantly, however, they were able to extrapolate that dehydration is more related to neuropathy (nerve damage) than complications in the vascular system.
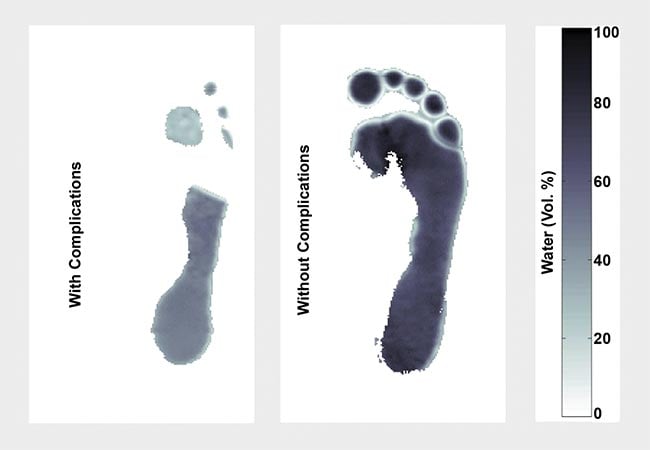
Figure 4. Exemplary water mapping images of two patients with diabetes with diabetic foot complications (left) and without complications (right). Courtesy of E. Castro-Camus.
Not that long ago, terahertz instrumentation was fit on a
board on an optical table, which made it impractical for in vivo use.
“Diabetic foot syndrome has two components: The high levels of glucose in the blood deteriorate the vasculature and the nerves over time (usually over many years), and this is particularly bad in the lower limbs,” Camus said. “When I started working on diabetic feet, as a physicist, I had a rather clear, and also naïve, mental image that the dehydration of the skin of the feet was a poor circulation problem. Dr. Irving Salas, a neurologist also working on our team, was really the person who was able to see the ‘big picture’ behind the data and realized that the true problem lies in the nerves … since nerves don’t work properly, the feedback mechanism that brings information back about the dehydration of the feet stops working.”
Though a sabbatical and the COVID-19 pandemic caused a pause in this work, Camus said he has recently communicated with Murillo-Ortiz and Salas and hopes to continue their research in the near future.
References
1. O.B. Osman et al. (2022). In vivo assessment and monitoring of burn wounds using a handheld terahertz hyperspectral scanner. Adv Photonics Res, Vol. 3, No. 5, p. 210095.
2. M. Khani et al. (2023). Triage of in vivo burn injuries and prediction of wound healing outcome using neural networks and modeling of the terahertz permittivity based on the double Debye dielectric parameters. Biomed Opt Express, Vol. 14, No. 2, pp. 918-931.
3. G.G. Hernandez-Cardoso et al. (2022). Terahertz imaging demonstrates its diagnostic potential and reveals a relationship between cutaneous dehydration and neuropathy for diabetic foot syndrome patients. Sci Rep, Vol. 12, No. 1, p. 3110.
Misconceptions in Terahertz
Terahertz imaging has potential advantages compared with other techniques, but some of what it can offer has, unfortunately, been exaggerated in literature, said Daniel Mittleman, a professor of engineering at Brown University. While Mittleman was a postdoctoral researcher at AT&T Bell Laboratories in the mid-1990s, he was instrumental in the development of advancements in terahertz spectroscopy and imaging on a team led by Martin Nuss.
One example of a potential advantage is its ability to outperform x-rays in certain environments, he said.
“Suppose you want to find air bubbles embedded in a foam insulation, for example, on the exterior surface of the Space Shuttle, or in the dashboard of a car that you manufacture,” he said. “X-ray imaging would be useless because the foam is 99% air, so it looks no different from the air bubble. But terahertz imaging works well because typically the size of the structures in the foam are near to the terahertz wavelength, so the edges of the foam scatter a lot, giving rise to huge contrast between the foam and the air bubble you are trying to find.”
But while the terahertz spectral range contains myriad physical and chemical properties that can be identified in materials, many biological samples are virtually opaque to terahertz imaging because of its strong absorption in water. Most studies of hydration are at the surface level, he said, which can be useful in some contexts.
“Measuring surface hydration of living tissue can be useful for several different things, although of course there are other ways to do this — i.e., MRI does a really good job at this task in some cases,” he said.
In a perspective published with fellow researcher Andrea Markelz, Mittleman argued that certain pervasive myths in the field have overshadowed the balanced research of some of his colleagues. They pointed to influential claims that terahertz waves can manipulate DNA molecules inside cells, and that many predictions of terahertz vibrational modes rely on calculations based on molecules in the gas phase, which is not indicative of a real-world biological environment. In addition, there have been misleading statements related to the ability of terahertz waves to heat samples effectively, beyond what could be accomplished with other, far less costly techniques1.
Mittleman and Markelz also mentioned positive research that has been completed in the terahertz regime, in the areas of cancer and burn imaging, diabetic foot syndrome, and water content of the cornea. Mittleman mentioned further advancements in materials science, such as the use of terahertz spectroscopy on single-atom defects in crystal as well as in terahertz communication.
“Terahertz time-domain spectrometers are just one way to access the terahertz range,” he said. “It happens to be my favorite way, and it may even be the most common in research labs around the world, but it is important to realize that there are many other ways. Time-domain terahertz spectroscopy has a lot of shortcomings in view of the needs of certain applications, so in many cases, you’d choose to use other methods to generate and detect signals.”
Reference
1. A.G. Markelz and D.M. Mittleman. (2022). Perspective on terahertz applications in bioscience and biotechnology. ACS Photonics, Vol. 9, No. 4, pp. 1117-1126.