As superresolution microscopy has advanced, so too has scientists’ ability to dive deep into the neural network and the compounds that accumulate in conjunction with a variety of conditions. And a team at the VIB-KU Leuven Center for Brain & Disease Research has seized on this technology to understand a protein complex that is common in patients with Alzheimer’s disease and with cancer.
The γ-secretase complex is an enzyme composed of catalytic presenilin (PSEN), nicastrin (NCT), anterior-pharynx defective 1 (APH1), and presenilin enhancer 2 (PEN2) in a 1:1:1:1 ratio. The compound breaks down when this ratio is not present.
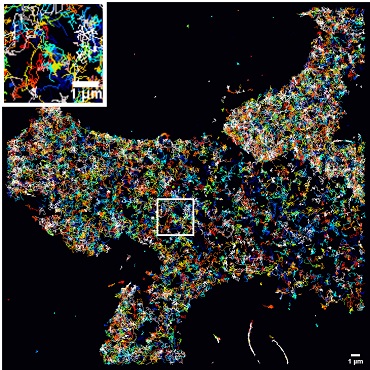
PSEN1/γ-secretase diffusion is fast at the cell surface. This is a representative cell showing single-particle tracking coupled to photoactivated localization microscopy tracks of mEOS3.2-PSEN1 rescued mouse embryonic fibroblasts. (Color scale = frame of track initiation; scale bar = 1 µm.) Courtesy of Abril Escamilla-Ayala.
“The γ-secretase complex is one of the two enzymes responsible for the production of toxic amyloid-beta, the main component of amyloid plaques, with the accumulation of amyloid plaques in the brain being a hallmark of Alzheimer’s disease,” said Sebastian Munck, the bioimaging core leader at Leuven. “Most of the mutations that cause familial Alzheimer’s disease are found in γ-secretase catalytic subunit, presenilin (PSEN).”
He added that the compound’s presence was not in and of itself negative.
“The four subunits are necessary for γ-secretase activity,” Munck said. “It is important to note that the γ-secretase not only processes the proteins that form the plaques but also has vital functions for the organisms. This is one of the reasons why designing safe drugs is difficult for that purpose.”
Studying the protein phenomenon was difficult with conventional microscopy, which was limited in its resolution.
According to the team’s research, in living neural cells, single-particle tracking photoactivated localization microscopy (sptPALM) showed that PSEN1/γ-secretase tagged with fluorescence gathered in “hot spots,” or high track-density areas sensitive to γ-secretase inhibitors. They used structure illumination microscopy (SIM), molecular counting by photobleaching, and sptPALM to track this protein at the plasma membrane.
The scientists found that although PSEN1/γ-secretase did accumulate in these hot spots, these groupings did not gather to the same extent near the cell’s edge, where the enzymes were more mobile. They believe as more is learned about the distribution and movement of these enzymes, the more their molecular functioning will be unlocked, opening the door to more effective treatment.
“We want to explore more the nature of γ-secretase hotspots and continue understanding regulatory mechanisms of γ-secretase dynamics,” Munck said. “Now that we have the basic characterization, we are working as well in the characterization of familial Alzheimer’s disease mutations happening in the subunit presenilin.”
This research was published in eLife (www.doi.org/10.7554/eLife.56679).