No/low compensation panels reduce complexity of immunophenotyping assays while generating high-quality data from unique signals.
Sharon Sanderson, Bio-Rad Laboratories
Flow cytometry is an analytical technique that enables rapid, high-resolution analysis of diverse cell populations at high throughput. In fields such as immunology, oncology, and drug discovery, the ability to identify different cell types from a test sample suspended in fluid accelerates breakthroughs in understanding disease mechanisms and effective therapies. By using antibodies conjugated to fluorophores, flow cytometry facilitates the detection and quantification of cell surface, intracellular, and nuclear markers across diverse cell populations.
Immunoglobulin, the most common type of antibody. The identification of antibodies through carefully designed panels makes flow cytometry useful for diagnostics. Courtesy of iStock.com/koto_feja.
Multiplexing assays in flow cytometry offer the ability to measure multiple markers simultaneously in a single experiment, providing a comprehensive view of complex cellular processes, such as proliferation. This approach is particularly valuable in cancer research, where scientists can analyze different immune cell phenotypes and activation states within a heterogeneous sample to inform personalized therapy strategies.
However, the reliability of results obtained using complex multiparameter assays depend heavily on effective panel design and the careful selection of fluorophores to maximize the information that is collected.
Fluorophore properties
Multiplexing in flow cytometry provides great potential for comprehensive cellular analysis but necessitates careful panel design to ensure experimental success. As each additional fluorophore-conjugated antibody is added to a panel, the complexity increases, introducing challenges that can affect resolution and data quality. This is primarily due to the excitation and emission profiles of fluorophores, which are the wavelengths at which they absorb and emit light.
A key challenge in multiplexing using conventional flow cytometers is spectral spillover, where the emission signal from one fluorophore overlaps into the detection range of another (Figure 1). Spillover reduces signal clarity, complicates data interpretation, and can compromise overall experiment quality. The extent of spillover depends on factors such as the brightness of the fluorophore and the overlap of its emission spectrum with others in the panel. Selecting high-quality fluorophores with minimal spectral overlap and properties such as exceptional brightness and stability is therefore critical to optimizing data quality.
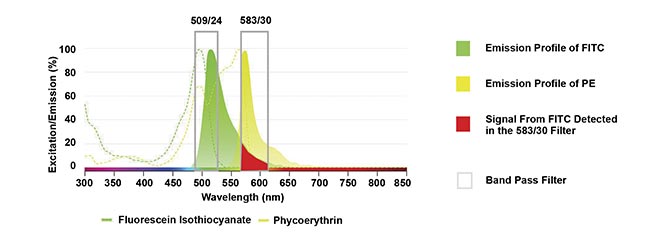
Figure 1. An example of spillover where the emission from fluorescein isothiocyanate (FITC) overlaps into the detector from the phycoerythrin (PE) filter. Courtesy of Bio-Rad Laboratories Inc.
To address spillover in conventional flow cytometry, fluorescence compensation is used, a process that corrects for overlapping signals, allowing the fluorescence detected in a specific channel to be accurately attributed to the intended fluorophore. This process relies on robust single-stained controls and careful calibration to account for the unique fluorescent characteristics of each fluorophore.
High spectral overlap and the subsequent need for compensation introduces additional challenges that may compromise data quality (Figure 2). Incorrect compensation, whether due to poor single-stained controls or manual errors, can result in over- or under-corrected signals, leading to inaccurate data. Furthermore, spillover contributes to data spreading, where compensated signals exhibit increased variability, masking dim signals and reducing detection sensitivity of a variety of cellular conditions1. This can significantly affect cell resolution, particularly for rare or low-abundance populations, and may lead to the misidentification of cell types. This can be overcome by careful panel design to avoid fluorophores with high spread or, if unavoidable, by using fluorophore pairs with high spread to detect mutually exclusive markers (Figure 2).
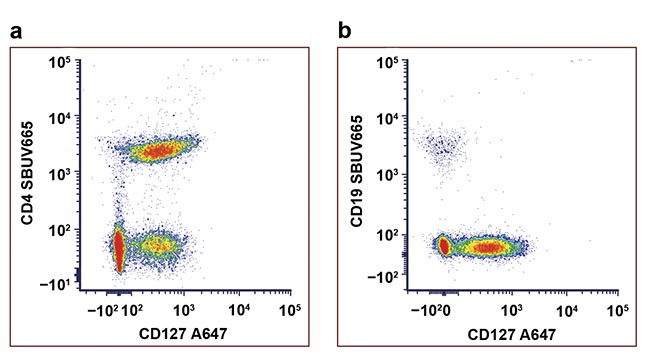
Figure 2. An example of data spreading. Staining of human peripheral blood with
antibodies conjugated to StarBright UltraViolet 665 (SBUV665) and Alexa Fluor 647 (A647) The CD4+ CD127− population cannot be resolved from the CD4+ CD127+ population (a). Using the same fluorophores to detect mutually exclusive markers avoids the issue of high spread (b). Courtesy of Bio-Rad Laboratories Inc.
No/low compensation panels
No/low compensation panel design overcomes the issues associated with spectral overlap by strategically selecting fluorophores that produce minimal or no spillover into adjacent detection channels. This approach offers clear advantages:
• Improved time-efficiency: Staining, acquisition, and analysis times are significantly reduced, accelerating research workflows.
• Simplified workflows: Panels are easier to implement and require fewer cells, which is particularly important for experiments involving limited samples (i.e., a small amount of biofluid).
• Cost savings: The lack of single-staining compensation controls translates to lower reagent costs.
• Clear unbiased data: Data manipulation is not required, and the minimal influence of other fluorophores ensures confidence that the signal is genuine. This is ideal when detecting markers of an unknown expression.
Successful no/low compensation panel design requires both technical expertise and careful planning. Adhering to best practices ensures optimal results by addressing factors such as sample quality, instrument configuration, fluorophore selection, and antibody titration2. Some of these best practices can be found in the minimum information about a flow cytometry experiment (MIFlowCyt) standard recommendations for published studies, which cover instrument approaches to instrumentation, assay development, and data processing3.
Sample quality and preparation
High-quality samples are key to achieving reliable flow cytometry results. Careful preparation not only enhances data accuracy but also prevents the need to repeat experiments, saving valuable time and resources.
Maintaining cell integrity is essential. Cells should be handled gently to avoid damage or artifacts that could affect results. When centrifuging, use appropriate speeds and avoid prolonged pelleting to minimize cell stress. Similarly, leave some supernatant when aspirating media to prevent cell dehydration.
Single-cell suspensions are crucial for successful flow cytometry. Cell clumps can block the cytometer, delaying cell data acquisition and potentially damaging the instrument. To prevent clumping, keep wash and media buffers at 4 °C when it is possible, and use appropriate dissociation techniques such as mesh filtering, enzymatic digestion, and tissue mincing.
Nonspecific cell binding should also be addressed to ensure success, because Fc receptors — proteins on the surface of certain immune cells, such as macrophages and monocytes — can cause nonspecific antibody binding, leading to reduced resolution and false positives. Adding Fc blocking reagents or host species serum can help mitigate this issue, e.g., using mouse serum for mouse cells. Including a viability dye in a sample is also critical when dead cells exceed 5% of the sample population to avoid false positives in the data.
Instrument configuration
Understanding a flow cytometer’s laser and filter setup is critical for effective panel design. Modern flow cytometers typically feature three or more lasers, each with detection filters covering distinct wavelength ranges. The number and specificity of lasers and filters determine how many fluorescent parameters can be analyzed simultaneously and which fluorophores are suitable for the instrument. By aligning the instrument’s capabilities with panel design, researchers can avoid unnecessary spectral overlap, optimize fluorophore selection, and ensure precise marker detection. All instruments are slightly different, and a panel that works on one may not be optimal for another.
Fluorophore selection
Choosing high-quality fluorophores with narrow, well-defined emission spectra is essential; this not only reduces compensation but also improves the resolution and accuracy of flow cytometry data that is essential to detect cell populations. In instruments with multiple lasers, using fluorophores with distinct excitation wavelengths, making them excitable by one laser, significantly reduces spillover by minimizing cross-laser excitation and signal into off-target detectors.
Pairing fluorophores with markers based on their expression profiles can further mitigate spillover. Bright fluorophores with high stain indices are ideal to detect markers with low levels of expression on cells, known as low-antigen density markers, or markers on rare cell subsets, such as when a disease is in its early stages. On the other hand, dimmer fluorophores are better suited for highly expressed markers. Another effective strategy to reduce spillover and spread includes employing fluorophore pairs with minimal spillover to identify mutually exclusive markers, such as the proteins CD3 and CD19. When dealing with cell subset markers present on the same cell type, or markers that show continuous expression patterns, it is essential to choose fluorophores that have little to no overlap.
Antibody titration
Proper antibody titration ensures optimal signal intensity is maintained while minimizing problematic background noise. Using excessive antibody concentrations can increase nonspecific binding, which risks the introduction of artifacts and false readings. Conversely, under-titrated antibodies may fail to effectively detect target markers. The stain index, which measures the separation between positive and negative populations and takes background signal into consideration, can be used to determine antibody concentration.
In addition to the integration of best practices for successful panel design, advancements in flow cytometry have addressed many of the limitations associated with the successful design of larger no/low compensation panels. High-parameter flow cytometers, such as the ZE5 Cell Analyzer from Bio-Rad Laboratories Inc., have expanded the number of markers that can be analyzed at the same time. These instruments incorporate sophisticated laser and filter configurations to enhance the detection of multiple fluorophores at high speed. This technology has significantly advanced drug discovery by enabling the efficient screening of novel molecules that produce desired biological activity.
In parallel, the development of next-generation fluorophores with enhanced properties has revolutionized panel design. For example, StarBright Dyes, also from Bio-Rad, offer narrow excitation and emission spectra, reducing spillover and improving data clarity. By contrast, some older fluorophores on the market, such as PerCP-Cy5.5, which has mid-level brightness, exhibit broad emission profiles and high cross-laser excitation, which increases compensation requirements and introduces spreading errors (Figure 3).
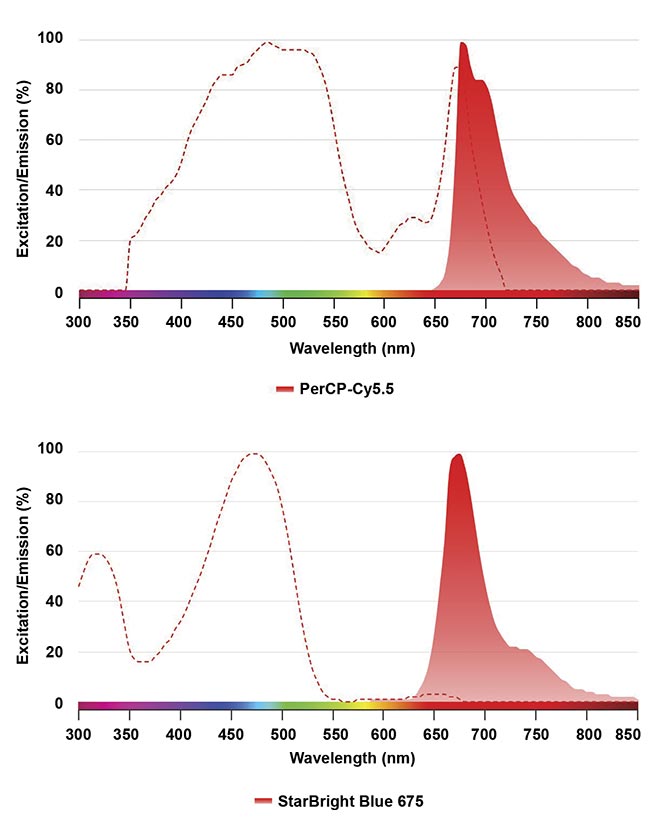
Figure 3. A comparison of spectral profiles between PerCP-Cy5.5 and StarBright Blue 675 using Bio-Rad’s fluorescence spectra viewer. The dashed line shows the fluorophore excitation profile, and the solid graph shows the emission profile. Courtesy of Bio-Rad Laboratories Inc.
Online tools, such as spectral viewers and panel-building platforms, have further simplified the process of designing panels, reducing the risk of selecting incompatible reagents and instrument settings. These advancements ensure that large, highly multiplexed panels maintain accuracy and efficiency, even when detecting rare cell types.
Human eight-color panel
While showcasing the capabilities of an eight-color low/no compensation panel design, a practical example was developed to aim to identify major cell populations within a human peripheral blood sample (blood from a healthy donor) using a five-laser cell analysis system (Figure 4). The no/low compensation panel design process began by identifying key markers, their expression patterns, and antigen densities. Adhering to panel design principles, antibodies against each marker were paired to a fluorophore and considered fluorophore brightness and antigen density while keeping spillover to a minimum.
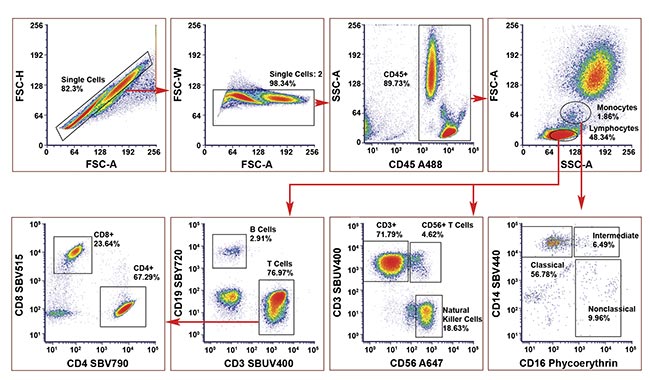
Figure 4. A human eight-color no-compensation panel on human peripheral blood to identify T cells, natural killer cells, and monocyte subsets. A488, Alexa Fluor 488; A647, Alexa Fluor 647; SBUV, StarBright UltraViolet; SBV, StarBright Violet; SBY, StarBright Yellow. Courtesy of Bio-Rad Laboratories, Inc.
For instance, CD45, a marker with high antigen density, expressed on all cells of interest, was paired with the dim fluorophore Alexa Fluor 488, ensuring clear resolution without overwhelming signal detection. The initial five fluorophores identified were each excited by a different laser and a spectra viewer tool was used to identify gaps where other fluorophores could be added with minimal spillover. Fluorophore pairs with minimal spillover were used to identify mutually exclusive markers.
The panel achieved minimal spillover, allowing for the accurate detection of all cell populations without compensation. A compensated and uncompensated version of the panel were compared as part of the validation process, which confirmed that compensation was not required because the same cell percentages were identified. The inward-tilted angles of the CD4+ and CD8+ populations in the lower right plot in Figure 4 suggests that some compensation could be applied; however, due to careful panel design and the use of bright fluorophores, these populations can still be accurately identified.
Path to greater precision
The ability to generate larger no/low compensation panels represents a significant advancement in flow cytometry, offering enhanced data quality, simplified workflows, and reduced costs. With the integration of advanced fluorophores and high-parameter instruments, more efficient panels can be achieved with minimal spillover, enabling accurate and precise cellular analysis. For instance, in clinical settings, even if the operator has limited knowledge of flow cytometry, robust and accurate results can be achieved to aid in the development of tailor-made therapies. As technological innovations in flow cytometry continue to advance, the future promises even greater precision, efficiency, and accessibility, empowering scientists to tackle some of biology’s most complex challenges with confidence.
Meet the author
Sharon Sanderson, Ph.D., is the product manager for flow regents and a flow cytometry applications scientist at Bio-Rad Laboratories Inc. She obtained a Ph.D. in cell biology from the University of London and has since gained more than 13 years of experience in flow cytometry from her work in academia and industry; email: [email protected].
References
1. R. Nguyen et al. (2013). Quantifying spillover spreading for comparing instrument performance and aiding in multicolor panel design. Cytometry Part A, Vol. 83A, No. 3, pp. 306-315.
2. H.T. Maecker and J. Trotter. (2006). Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry Part A, Vol. 69A, No. 9,
pp. 1037-1042.
3. J. Lee et al. (2008). MIFlowCyt: The minimum information about a flow cytometry experiment. Cytometry Part A,
Vol. 73, No. 10, pp. 926-930.