
Scientists Confirm Dynamin Molecular Motor’s Constriction Mechanism
A team at Kanzawa University used the measurement technique of single-molecule fluorescence resonance energy transfer, or smFRET, as well as a computable model, to develop a method that determined the strength of an individual dynamin motor. Dynamin, which refers to a family of biomolecules, is a protein that is a central player in endocytosis — a process that mediates the entry of diverse particles into cells, from nutrients to viruses. Its primary activity is to use guanosine triphosphate as fuel to constrict and cut membrane tubes.
However, the key quantitative aspects of dynamin’s function remain unclear.
smFRET enables measurement of distances in biomolecules in the nanometer range. Specifically, smFRET is the application of FRET within a single molecule. FRET is the process of energy transfer between two chromophores, or molecules that are sensitive to light. The efficiency of the energy transfer is inversely proportional to the sixth power of the distance between the chromophoric parts, which makes FRET very sensitive to changes in distance.
With its computable (or “computational”) model to resolve individual motors, and demonstration that dynamin produces sufficient force to tightly constrict a membrane tube when most of its motors are simultaneously cooperating, the researchers validated the prevailing “constriction-by-ratchet” model for the dynamin “nanomuscle.”
This “nanomuscle” is nature’s strongest torque-generating motor, the researchers said.
Via the process of endocytosis, cells in the human body constantly receive substances from outside the body. One endocytosis pathway involves the formation of a protrusion within the cell membrane, pointing to the inside of the cell, triggered when a molecule needing to “get in” reaches the membrane. The protrusion wraps and closes around the molecule, after which it is “cut off,” leaving the wrapped molecule (a so-called vesicle) inside the cell. Dynamin is a protein that can locally constrict cell membranes and chop off bits.
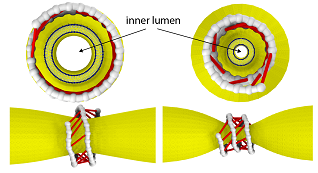
The filament coils around a membrane tube and, under GTP hydrolysis, forms active cross-bridges that collectively constrict the membrane. Courtesy of Kanzawa University.
Dynamin consists of helical filaments, which coil around the neck of a vesicle forming within a membrane. In the presence of GTP, a molecule that can be thought of as biochemical “fuel,” it provides energy as the filaments begin to slide and constrict the vesicle neck.
The researchers’ use of smFRET allowed them to gain insights into this constriction mechanism. Data showed that different conformations of dynamin’s filaments occur, depending on whether they are bound to GTP. These various configurations result in the formation of differently oriented molecular “bridges” between neighboring coil turns.
When the researchers supplied GTP and hydrolyzed it, they induced power strokes in the so-called cross-bridges and generated a filament torque.
The scientists then designed their computable model of a set of dynamin filaments wrapped around a membrane protrusion. This captured the structural changes and their timescales as observed in the smFRET measurements. The membrane was modeled as a deformable cylindrical tube, and the filaments were modeled as strings of beads that connected to active cross-bridges that were powered by GTP.
Simulations showed that the combined motion of cross-bridges provides a force large enough to cut a membrane tube, which confirmed the constriction-by-ratchet model of the dynamin “nanomuscle.”
The research was published in PNAS (www.doi.org/10.1073/pnas.2101144118).
Published: September 2021