Researchers from the Institute of Photonic Sciences (ICFO) have successfully demonstrated holographic Raman microscopy. The technology is poised to support wide-ranging applications, from live cell and tissue interrogation to, potentially, anti-counterfeiting.
Because spontaneous Raman scattering is more than 10 orders of magnitude weaker than fluorescence, the technique is rarely employed. However, Raman can be enhanced dramatically on metal surfaces or in metallic nanogaps to surpass fluorescence response; probes of nanometric surface-enhanced Raman scattering (SERS), therefore, are promising candidates for biological sensing applications, because they avoid molecular distortion despite their effectiveness depending on the size, stability, and brightness of individual particles.
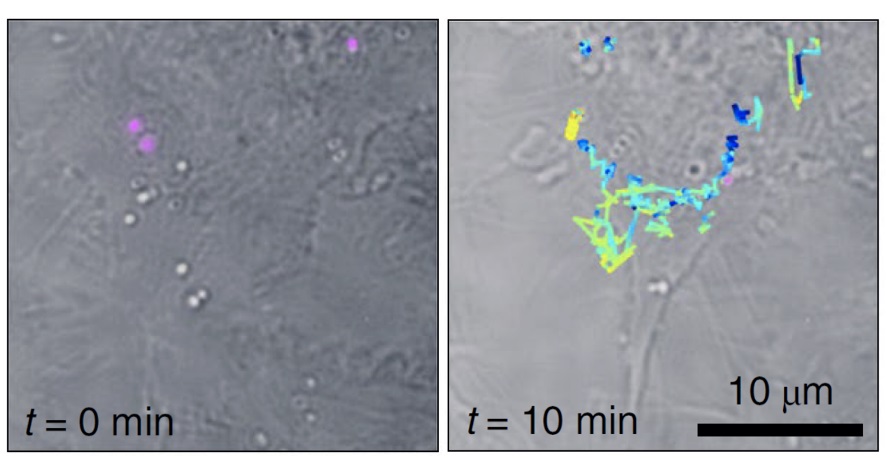
Tracking of live-cell SERS individual particles. The tracks of each of the particles are color coded to show the respective z-positions within the volume. Courtesy of ICFO/URV.
The method proposed by the ICFO researchers overcomes key issues with SERS-probe-based imaging. According to the researchers, the work began with a single question: “Can we make single SERS photons self-interfere?”
The problem is as follows, the researchers told Photonics Media: You have an image of your sample as you would see it with any normal wide-field microscope. The image is composed of incoherent light, photons generated by spontaneous Raman scattering. To gain extra information, the observer would need to measure the phase.
However, because the light is incoherent, an observer can’t compare it to another photon as a reference. Because incoherent photons exhibit random phase, a potential interference signature would vanish.
The only way to obtain phase information is to use individual photons as their own reference, though the phase term vanishes if one interferes two waves with identical phase.
To solve this problem, researchers working in the groups of ICREA professors Niek van Hulst from ICFO and Ramon Alvare-Puebla from Rovira i Virgili University synthesized plasmonic superclusters from small nanoparticle building blocks, generating very strong electric fields in a restricted cluster size. These SERS nanoprobes are extremely bright and require very low illumination light exposure in the near-infrared, thereby reducing the potential of photo-damaging live cells and enabling wide-field Raman imaging.
The researchers achieved self-interference through a shearing-interferometer that uses a 2D grating to generate four image copies, which are recombined on a camera via a relay imaging system. Each individual photon travels all possible paths through the interferometer and ultimately self-interferes on the camera.
The arrangement allowed the researchers to measure the phase-derivatives of the image, by slightly moving the grating; the four image copies no longer perfectly recombine but arrive on the camera with slight offsets. That implementation directly accessed the phase-derivative that one can integrate to obtain the real phase.
The researchers demonstrated Fourier transform Raman spectroscopy of the wide-field Raman images and localized single SERS particles in 3D volumes from a single shot. They then used those capabilities to identify and monitor single SERS nanoparticles inside living cells in three dimensions.
To further develop the technology, the researchers are designing next-generation SERS probes for intracellular concentration mapping and are adopting the shearing interferometer for multiplexed SERS-imaging. They are also preparing multiplex arrays for fast diagnosis optical devices.
The research was published in Nature Nanotechnology (www.doi.org/10.1038/s41565-020-0771-9).