A research project aims to realize deeper optical imaging techniques for cancer diagnosis and monitoring of the human body.
REGINA GUMENYUK, TAMPERE UNIVERSITY; PABLO LOZA-ALVAREZ, THE INSTITUTE OF PHOTONIC SCIENCES (ICFO); AND SIMONE MORSELLI, UNIVERSITY OF FLORENCE
Optical imaging of tissues in diagnostics is a burgeoning field of research, and related technology has shown the ability to provide rapid, sensitive, and specific data that discriminates disease-related characteristics. However, the depth at which high-quality imaging can be performed in tissue by existing systems has historically been limited to only a few hundred microns. This hinders examination and detection of deeper disease markers or morphologies.
Recent results in multiphoton imaging suggest that three-photon imaging with excitation at a wavelength of 1700 nm has a penetration depth that is 3× that of the equivalent two-photon imaging1 when searching for signs of disease. At this longer wavelength, loss coefficients due to scattering are much lower. Rayleigh scattering varies as the inverse fourth power of the wavelength, and Mie scattering varies as 1/λn, meaning a total of 5× less scattering compared with 1-μm wavelength for soft tissue. Higher-contrast images should also be achievable, given a signal-to-noise ratio of around 5 dB at 1700 nm — as compared to a ratio of 0 dB at 1300 nm — for 1.1-mm penetration depth.
A conceptual image of a bladder with microscopic malignant cells spreading throughout the human body. Courtesy of Lightspring/Shutterstock.com.
Although absorption is higher, there is a window of lower absorption between 1550 and 1870 nm. Scientists have termed this largely unexplored spectral region the “third optical window”2. This spectral window has previously gone largely unstudied due to a higher water absorption level than in the first or second biological windows. However, nonlinear absorption effects specific to the third biological window result in faster intensity reduction of scattered fundamental light than the generated fluorescent signal3. This phenomenon allows for improvement of the imaging contrast by distinguishing between the absorption of scattered (diffused) light and signal (ballistic) light. This concept was also confirmed and demonstrated in the laboratory, by deep high-contrast imaging of the mouse brain with a 1680-nm short-pulse laser source1.
Considering the potential for increased penetration depth and the reduced effect of optical scattering, these longer wavelengths are, in principle, favorable for both the excitation and emission produced in photonics imaging systems. However, there is currently a lack of suitable laser sources to deploy in imaging technologies addressing this third biological window.
A multidisciplinary team of European scientists launched a research collaboration called AMPLITUDE (Advanced Multimodal Photonics Laser Imaging Tool for Urothelial Diagnosis in Endoscopy) last year to develop new fiber laser sources for an innovative multimodal medical imaging system that will produce earlier diagnosis and improved monitoring of bladder cancer treatment. The international team will create unique lasers that will generate light in previously unavailable infrared regions and penetrate much deeper into tissue, where the early signs of cancer’s presence often lie undetected. By combining the lasers with multiple detection techniques, the integrated imaging platform, designed for use in microscopy or endoscopic probes, will be able to capture high-resolution images at depths up to 3× greater than existing clinical diagnostic tools (Figure 1).
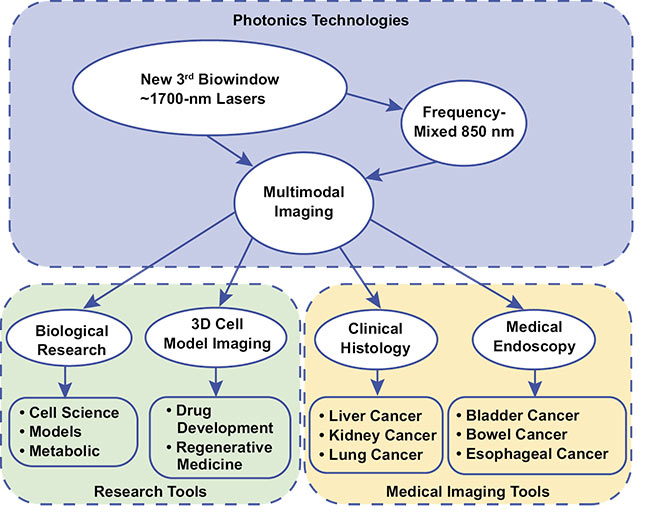
Figure 1. An illustration of the biowindow-targeting sources and the multimodal-imaging approach of the AMPLITUDE Project. Courtesy of MODUS.
Monitoring bladder cancer
Diagnosing how deeply a tumor reaches into normal tissue is crucial for selecting the optimal treatment strategy — including degree of invasiveness, the depth of resection, and the proper post-treatment procedure — in patients with bladder cancers. Urothelial cell carcinoma is the 11th most commonly diagnosed cancer worldwide, with an age-standardized incidence rate per 100,000 people of 9.0 for men and 2.2 for women. Urothelial carcinomas are also one of the costliest lifetime cancers per patient to treat, as the cancers have a high recurrence rate of more than 50% within five years. This means patients must be constantly monitored for potential recurrence.
As with other cancers, early diagnosis means there is greater chance of survival. However, a lack of accurate and reliable diagnostic tests means that bladder cancer is often diagnosed late, with a comparatively high percentage of patients diagnosed only upon emergency admission, resulting in recurrence of cancer or even death. Increasing the resolution and penetration depth would enable doctors to quickly and accurately identify and monitor bladder tumors, helping them to more reliably diagnose and treat patients earlier in the progression of their disease.
Pulsed-laser sources
A widely tunable, mode-locked fiber laser capable of producing subpicosecond pulses between 1700 and 1800 nm to achieve the requisite resolution has been demonstrated by a group at Tampere University4. This laser employs a hybrid mode-locked technique, comprising an acousto-optic tunable filter as a frequency shifter together with nonlinear polarization rotation in the cavity. Notably, the acousto-optic filter also allows precise electronic tuning of the wavelength. These results create a platform for the development of tunable ultrafast thulium-doped and Raman fiber lasers at the wavelengths necessary to access imaging in the range of 1650 to 1800 nm for cancer diagnosis.
The development of nonlinear techniques such as frequency shifting by Raman effect and four-wave mixing makes it possible to go beyond the traditional rare-earth dopant’s performance envelope (the use of ionization to intensify lasers), expanding the pulsed laser to new wavelengths and allowing nonlinear imaging of tissues (Figure 2).
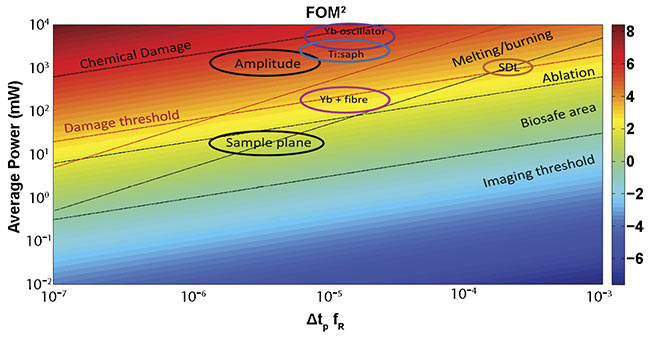
Figure 2. The figure of merit (FOM) corresponding to a microscope objective with an NA = 1 for a two-photon process. The area surrounded by bold ellipses shows the attractive characteristics for the AMPLITUDE laser (10 MHz, 600 fs at 1700 nm) and the target specs in the sample plane. The color scale is logarithmic. SDL: semiconductor disc laser; Yb: ytterbium. Courtesy of ICFO.
An attractive way to develop a compact, efficient, and low-cost ultrashort-pulse-laser source is second-harmonic generation. Periodically poled nonlinear crystals contain a waveguide that, due to the high-pump-light intensities over a long interaction length, allows highly efficient frequency conversion, even at low and moderate pump power levels. Periodically poled lithium niobate (PPLN) waveguide crystal offers high nonlinearity along with strong damage thresholds, presenting highly efficient nonlinear wavelength conversion crystal. PPLN is used for frequency doubling, difference- and sum-frequency generation, optical parametric oscillation, and other nonlinear processes.
New PPLN for 1700/850 nm broadband second-harmonic generation will be developed for medical imaging by the Amplitude Project. This will provide significant advantages, including application convenience, by integrating the frequency-mixing device into a convenient, compact, robust, and cost-effective packaged format.
Multimodal microscopy that combines confocal and multiphoton techniques — including two- and three-photon excited fluorescence and autofluorescence, and second- or third-harmonic imaging techniques — can now theoretically be feasibly targeted for clinical deployment (Figure 3). By integrating other technologies such as Raman and metabolic imaging, the prospects for identifying specific biomarkers are significantly increased.
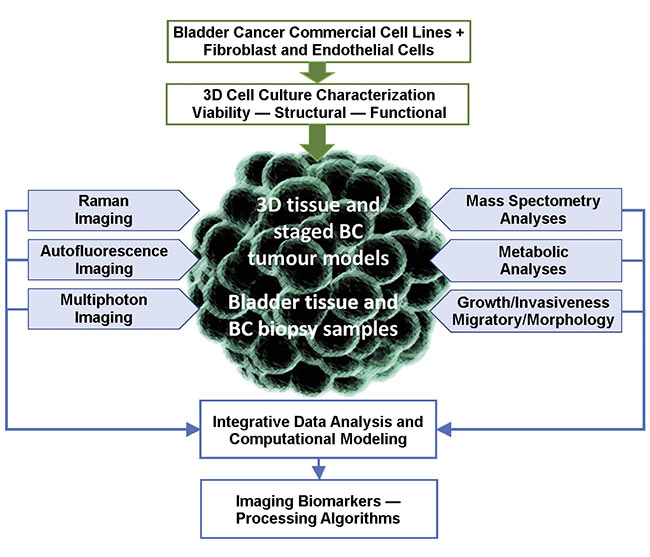
Figure 3. A cell model and ex vivo tissue imaging, and its biological correlation (BC) with imaging biomarkers. Courtesy of MODUS and UNIMIB.
By using the frequency-doubled source that reaches 850 nm as a replacement for the “workhorse” Ti:sapphire laser typically used for nonlinear imaging, cellular morphological information can be derived by means of an efficient deep-tissue contrast mechanism from unstained tissue. This autofluorescence can provide important functional information about the tissue by focusing on specific molecules involved in metabolism.
By detecting the signal within specific emission bands using a ratiometric or time-resolved approach, it is possible to optically detect the oxidative stress of a tissue, providing important information about tissue metabolism, which is strictly related to dysplasia and neoplasia. The three-photon excited fluorescence signal can also be directly generated by direct-focusing the fundamental laser at 1700 nm or by frequency mixing with the frequency-doubled signal at 850 nm. This signal can be used for extracting relevant functional information from tissue, including more specific targeting of NADH (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide) fluorophores, enabling higher molecular imaging contrast and increased sensitivity of metabolic conditions.
Translating results to clinic
Benchtop microscope systems for histology labs are high-end instruments typically used for research and routine applications in medicine and industry. Because these systems are fixed in a laboratory, they can be equipped with advanced automation, tilting objective, data collection, calibration mechanisms, and sophisticated visualization software. One platform suitable for implementation of multimodal tissue imaging for histology is the Femtonics FEMTOSmart dual microscope that provides the ability to perform dual scanning by using both galvo and resonant scanners in tandem.
With a galvo scanner, it is possible to zoom in on tiny structures such as squamous cells and move quickly between these regions. In contrast, with a resonant scanner it is possible to capture images using a high frame rate to follow rapid changes in the field of view. Integrating the laser sources into one platform will enable multiphoton imaging with deep penetration depth. When this capability is combined with Raman and fluorescence imaging at 850 nm, it can provide unparalleled specificity and sensitivity in a microscope system, offering breakthrough diagnostics and therapy-monitoring possibilities.
Multimodal imaging endoscopy
Endoscopy is one of the most important diagnostic and monitoring modalities in the management of cancers that are accessible via body cavities. Health care providers use endoscopy to review many parts of the human body with minimal to no invasiveness. Advancements in imaging have progressively added new tools for endoscopists, with the goal of achieving more accurate, sensitive, and accessible visual diagnoses for the benefit of patients with cancer. Today, many varieties of endoscopes use modern optical fiber-based systems such as those developed by LEONI, as shown in Figure 4.
Figure 4. The optical fibers used in LEONI medical devices. Courtesy of LEONI.
Endoscopic fluorescence confocal microscopy has been performed with reusable miniaturized imaging probes that range from 0.85- to 2.6-mm diameters and are compatible with working channels of standard cystoscopes. Real-time endoscopic microscopy of normal urothelium and low- and high-grade urothelial carcinoma have been demonstrated in the literature, with images comparable to conventional histopathology.
Similarly, in vitro studies using multiphoton microscopy have provided high-resolution images from fresh bladder tissues that are comparable to histopathology images for differentiating between malignant and benign features of gastric tumors. But limited depth of penetration using a classic Ti:sapphire laser source was unsuitable for cancer staging and lacked nuclear morphology on the images.
To realize the potential for endoscopic multimodal imaging, new solutions are needed in fiber lasers, from both researchers and industry, for different wavelengths and appropriate dimensions involving various fiber, buffer, and jacket materials, along with appropriate numerical apertures. Probes with application-specific adaptations are needed, such as distal and proximal tip design, with the diameters and lengths necessary to aid clinicians in their work.
Meet the authors
Regina Gumenyuk, Ph.D., is a senior research fellow at Tampere University in Finland and the coordinator of the AMPLITUDE Project. Her expertise includes design, fabrication, and characterization of ultrafast fiber lasers, high-power fiber amplifiers, and chirped pulse amplification systems; email: [email protected].
Pablo Loza-Alvarez, Ph.D., leads the Super-resolution Light microscopy and Nanoscopy (SLN) lab at the Institute of Photonic Sciences (ICFO), which develops novel multimodal light microscopy techniques and specializes in superresolution-imaging light sheet, multiphoton, and confocal microscopy; email: [email protected].
Simone Morselli, M.D., is a physician and clinical researcher at the University of Florence in the Unit of Urological Robotic Minimally Invasive Surgery and Renal Transplantation at Careggi Hospital in Florence; email: [email protected].
Acknowledgments
The Amplitude Project is coordinated by Tampere University (Finland) and comprises several specialists from across Europe, including the Aston Institute of Photonic Technologies (AIPT) (U.K.), MODUS Research and Innovation Ltd. (U.K.), Consiglio Nazionale delle Ricerche (CNR) (Italy), the University of Milano-Bicocca and the University of Florence (Italy), the Institute of Photonic Sciences (ICFO) (Spain), Ampliconyx Oy (Finland), Femtonics Ltd. (Hungary), HC Photonics Corp. (Taiwan), and LEONI Fiber Optics GmbH (Germany). The Amplitude Project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 871277.
References
1. N. Horton et al. (2013). In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat Photonics,
Vol. 7, pp. 205-209, www.doi.org/10.1038/nphoton.2012.336.
2. L. Sordillo et al. (2014). Deep optical imaging of tissue using the second and third near-infrared spectral windows. J Bio Opt, Vol. 19, No. 5, p. 056004.
3. K.L. Yoo et al. (1991). Imaging through a scattering wall using absorption. Opt Lett, Vol. 16, pp. 1068-1070.
4. T. Noronen et al. (2016). Electronically tunable thulium-holmium mode-locked fiber laser for the 1700-1800 nm wavelength band. Opt Exp, Vol. 24, pp. 14703-14708.