NAGANO, Japan, Aug. 18, 2020 — The breakdown of water has long been conceived as a clean way to produce hydrogen fuel, but the process has proven to be inefficient for widespread energy production in this manner. But the work of a team of scientists from Shinshu University and the University of Tokyo in Japan just might be cutting through that limitation.
According to researchers, the quantum efficiency of water splitting with typical photocatalysts is very low, resulting in a disappointing energy conversion rate. But according to their published research, they achieved a rate of around 96% efficiency, in the 350- to 360-nm range, using a new method of solar water splitting.
Tsuyoshi Takata, a professor at the Centre for Energy and Environmental Science at Shinshu University, said historically, a variety of inorganic semiconductors had been used as photocatalysts. Recently, the focus has shifted to oxides.
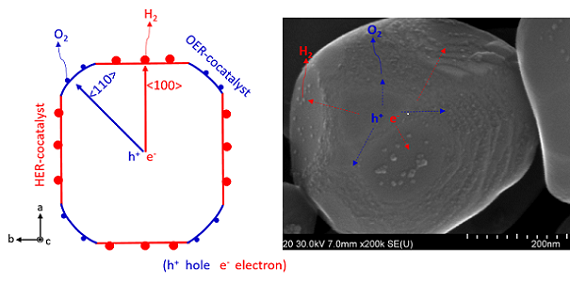
A model of an anisotropic charge transport (left) and an SEM image of a photocatalyst. Courtesy of Tsuyoshi Takata et al./Shinshu University.
In the process of solar water splitting, sunlight is directed toward suspended light-activated semiconducting particles. In past processes, the electron dispersion proved a difficult challenge to overcome. The team settled on strontium titanate (SrTiO3) as a photocatalyst, with additional cocatalysts applied in select areas to reduce backward transfer of electrons, and used aluminium doping to reduce defects in the crystal lattice.
“Photoexcited electrons and holes are separated in different directions in each photocatalyst particle,” Takata explained.
These improvements were confirmed by investigation through scanning electron microscopy and energy-dispersive x-ray spectroscopy. “Scanning (transmission) electron microscopy and EDS were utilized for revealing the photocatalyst structure,” Takata said.
For a light source, the scientists used a xeon lamp, which is similar to the sunlight spectrum. “Part of the experiment was conducted using a simulated sunlight,” he said.
The authors acknowledged that SrTiO3 may not be practical to use on a widespread basis in the field, since it is best used for absorbing near-ultraviolet light as opposed to visible light. But nevertheless they had demonstrated what was possible in increasing the quantum efficiency of solar water splitting with the aid of specially designed photocatalysts.
“The next target is to develop narrow band-gap photocatalysts to sufficiently absorb visible light,” Takata said.
This research was published in Nature (www.doi.org/10.1038/s41586-020-2278-9).