By leveraging the imaging contrast created in the light-matter interactions caused by laser excitation, photoacoustic remote sensing provides detailed information in real time.
Parsin Haji Reza, University of Waterloo
As microscopic optical inspection techniques have progressed over the years, they have provided valuable insights into the composition, structure, and function of cells and subcellular structures, transforming the way researchers and clinicians look at human tissues. Today, thanks to the refinement of photoacoustic microscopy, clinicians and researchers can visually assess cellular-level structures and functional information to better understand cancer and inspect the inner workings of living eyes to evaluate the root causes of blinding diseases.
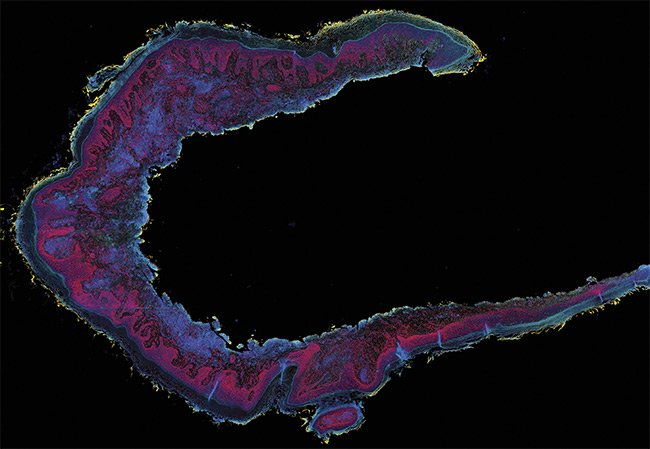
A second-generation photoacoustic remote sensing (PARS) image of a resected human skin tissue section exhibiting squamous cell carcinoma. Nuclei (red and pink). Connective tissues (blue, green, yellow). Courtesy of PhotoMedicine Labs.
Other technologies have also advanced the exploration of cellular dynamics. Fluorescence microscopy has enabled live-cell observation, illuminating the structure and measurement of biomolecules and highlighting specific interactions between mitochondria and DNA within cells. Optical coherence tomography (OCT) has quickly become the gold standard for ocular imaging, enabling visualization of the retina and retinal nerve fiber layer, facilitating our understanding of healthy and diseased eyes.
A more recent development, photoacoustic microscopy enables users to leverage optical absorption contrast, which provides information about chemical bonds, molecular structure, blood oxygenation, and other biochemical data. It is a label-free technique (meaning it does not require external agents) that penetrates scattering media to provide high-spatial-resolution images of a wide variety of light-absorbing chromophores, including RNA, DNA, hemeproteins, and lipids.
Photoacoustic microscopy is an advanced hybrid technology that functions by using a narrowband, pulsed excitation laser to target the optical absorption peak of specific chromophores and provide structural, functional, and molecular information. When the excitation laser energy is absorbed by targeted chromophores, the chromophores produce thermoelastic expansion that generates vibrations (ultrasound waves) that travel to the sample surface where they can be detected by an ultrasonic transducer.
Despite being a powerful imaging tool for several applications, such as measuring blood flow dynamics and oxygen metabolic rates in early cancer diagnoses, photoacoustic microscopy has important limitations: It requires contact with the sample through a coupling medium and ultrasonic transducer, and it is limited by the speed it takes for ultrasound waves to move through the sample to the surface, where it can be detected (time of flight).
In 2017, the author, along with other researchers, reported the first noncontact, noninterferometric photoacoustic remote sensing (PARS) technique1. Akin to photoacoustic microscopy, PARS uses laser excitation to generate thermoelastic expansion. Unlike the contact-based method, however, PARS systems function remotely by implementing another co-focused detection beam to recover the absorption-induced modulations of each chromophore as backscattered intensity variations. PARS’ noncontact architecture enables thermoelastic expansion to be measured directly at the source with maximal sensitivity, increasing speed both by avoiding ultrasound time-of-flight delays and by enabling the use of excitation lasers, with a pulse repetition rate up to
10 MHz or more in some applications.
To date, PARS has achieved a penetration depth of up to 2.5 mm in scattering gel-based tissue phantoms2, and it can be used where contact with a sample is impractical or the working space and footprint are restricted — for example, in endoscopy and surgery. Moreover, remote detection opens up several important applications by reducing the potential for contamination, deformation of the tissues due to contact, and patient discomfort if used in situ. PARS can function both interferometrically or noninterferometrically, extending its applicability. Finally, its all-optical architecture enables it to be highly compatible and easily integrated with other optical imaging modalities, such as OCT.
The technology advances
In 2018, the author founded his research lab, PhotoMedicine Labs, at the University of Waterloo, with the goal of advancing the PARS technique for multiple applications in medical research. His team has made extensive improvements to PARS architecture, optimizing the technique for multiple applications, including histology and ophthalmology. In that context, a second-generation technique was initiated that shifts its focus from detecting only photoacoustic signals to visualizing a full range of optical absorption and scattering contrasts. This second-generation technique has the potential to provide more detailed information in real time, with the high sensitivity and resolution necessary for clinical use.
Second-generation PARS leverages most light-matter interactions — including optical scattering, radiative relaxation, and nonradiative relaxation — in a single acquisition to provide rapid, remote, high-resolution optical absorption and scattering visualizations of biological tissues. This is the first time that an independent imaging modality has provided these three imaging contrasts in a single acquisition, which could yield additional information for future research3.
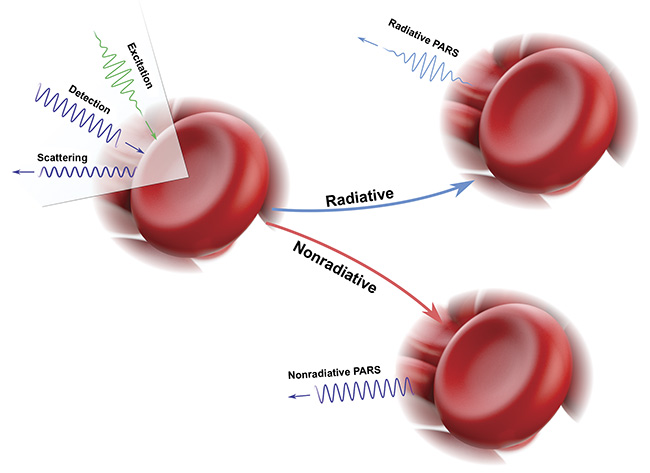
Second-generation PARS contrast mechanisms generated by the excitation laser. Nonradiative relaxation leads to heat- and pressure-induced modulations, both of which cause back-reflected intensity variations in the detection beam. The radiative absorption pathway captures optical emissions attributed to radiative relaxation (for example, autofluorescence), and the local scattering contrast is captured as the unmodulated backscatter (preexcitation pulse) of the detection beam. Courtesy of PhotoMedicine Labs.
This iteration of PARS functions by focusing a picosecond- or femtosecond-scale pulsed excitation laser into biological tissues to generate radiative relaxations (optical emissions), nonradiative relaxations (heat and pressure), and scattering effects from both excitation and detection lasers in the sample. Absorbed photons are captured by chromophores and converted into different forms of energy that are released from the sample as nonradiative and radiative relaxations, while scattered photons continue moving and interacting with other portions of the sample. Nonradiative relaxations are recorded using a secondary confocal interrogation beam co-focused and co-scanned with the excitation spot, enabling temperature and pressure changes (photoacoustic signals) to be detected as close to the source as possible. These changes are registered as modulations in backscattering intensity, which are then directly correlated to the local nonradiative absorption contrast.
Like traditional PARS technology, second-generation PARS also functions as a noncontact system capable of instantaneous detection of induced modulations in the excited sample. The unperturbed backscatter (preexcitation event) simultaneously captures the scattering contrast. The secondary detection pathway is used to capture radiative relaxation contrast. The radiative detection pathway was designed to exclude the excitation and detection pathways but collect all other optical emissions at every wavelength. These nonspecific optical emissions could include signals attributed to multiple radiative effects — for example, Raman scattering, stimulated Raman scattering, autofluorescence, or multiphoton autofluorescence.
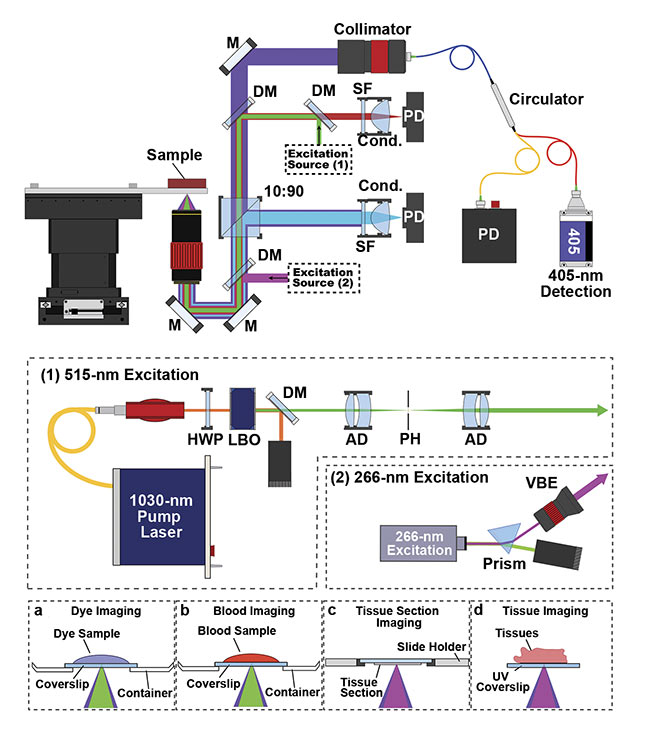
A simplified experimental system setup using a 266-nm excitation system. Configurations at the bottom
(a-d) show how various specimens would be prepared for imaging. M: mirror; DM: dichroic mirror;
SF: spectral filter; PD: photodiode; Cond.: condenser lens; HWP: half-wave plate; LBO: lithium triborate crystal; AD: air-spaced doublet; PH: pinhole; VBE: variable beam expander. Courtesy of PhotoMedicine Labs.
PARS offers a noncontact modality that facilitates the imaging of delicate samples and could extend the range of applications to those in which contact is impractical or space is limited, such as in situ histological imaging and accurate functional and structural ocular imaging.
Histology applications
Postoperative histological analysis of surgical margins is the gold standard for cancer margin assessment after surgery, but the histological workflow is time- and resource-intensive, and it can take several days to obtain results. Although negative (cancer-free) margins are one of the strongest indicators of surgical success and favorable patient outcomes, it is currently very difficult to determine exactly how much tissue around the tumor to remove, due to a lack of identifiable specificity in the tissue. As a result, positive surgical margins, which indicate that cancer cells remain present at the edge of the resection specimen, still occur in up to 30% of initial resections4 and can potentially have devastating impacts on patient health, as well as adversely affect a patient’s prognosis. Positive surgical margins frequently require reexcision surgery and adjuvant treatments, increasing health risks, health care costs, and stress for patients.
A primary goal of the author and his team has been to create a PARS system capable of imaging unprocessed freshly resected tissues, and of in situ histological imaging that could then be integrated into an advanced surgical microscope to guide complete cancer resection at a cellular level during the surgery. Modern PARS methods have enabled high-resolution, noncontact direct imaging of both DNA and cytoplasmic structures, creating an exact virtual replication of the hematoxylin and eosin (H&E) staining pattern that pathologists are already trained in. This will ease the transition of PARS histological imaging into the clinical workflow.
PARS leverages radiative relaxation to directly image individual chromophores and emulate eosin contrast while also using the excitation to target the optical absorption peak of DNA and image the nuclear structures normally stained with hematoxylin. With both radiative and nonradiative imaging contrasts, an exact virtual replication of the information provided by gold-standard H&E images can be generated without the need for staining or tissue preparation, and in a single acquisition.
To improve the sensitivity of PARS for histology and facilitate the detection of radiative absorption contrast, systematic changes have been applied. This includes using a picosecond 266-nm excitation laser to generate both radiative and nonradiative contrasts. A 405-nm detection laser has been used to improve scattering resolution and the confocal overlap of the PARS excitation and detection (for nonradiative absorption contrast) parts of the sample. To isolate and detect the radiative absorption contrast, a specific optical pathway with dichroic filters and an avalanche photodiode has been implemented.
These modifications, along with implementing a fully fiber-based probe beam pathway and avalanche photodetector, improve the sensitivity of this iteration of PARS over previous systems. Implementing a visible wavelength probe improves flexibility between the visible and UV excitation wavelengths. By reducing the disparity in the excitation and detection wavelengths, chromatic aberrations were suppressed when using refractive optics, providing clearer contrast.
Developers of the PARS histology system are now working to improve imaging speed by implementing faster megahertz pulse-rate excitation lasers along with a hybrid optical-mechanical and line-scanning process. These alterations should enable the system to achieve scan rates of less than 2 min/cm2 at 500-nm resolution, allowing an entire 2- × 2-cm tissue section to be imaged in less than 5 min.
This system is currently undergoing clinical trials for applications in skin and breast cancer, and once it is approved, it will be developed into the first tabletop histology system to achieve gold-standard H&E images directly from freshly resected tissue. This will be the first PARS product to be commercialized by the author’s startup, illumiSonics Inc. The team will then work toward the ultimate goal of creating a real-time surgical histology microscope by increasing the system’s imaging speed and modifying the system to enable safe in situ imaging.
Ophthalmology applications
For most common blinding diseases — such as age-related macular degeneration, diabetic retinopathy, and glaucoma — functional changes occur prior to structural changes, making their early detection crucial to understanding pathogenesis, early diagnosis, and timely management of ophthalmic disorders. Recently, multiwavelength PARS microscopy was introduced to provide noncontact imaging of melanin concentration in the retinal pigment epithelium; to measure the thickness of the retinal pigment epithelium; to measure ocular blood flow down to a single capillary, or even image an individual red blood cell; and to enable direct measurements of functional details such as oxygen saturation (SO2) and metabolic rate of oxygen (MRO2).
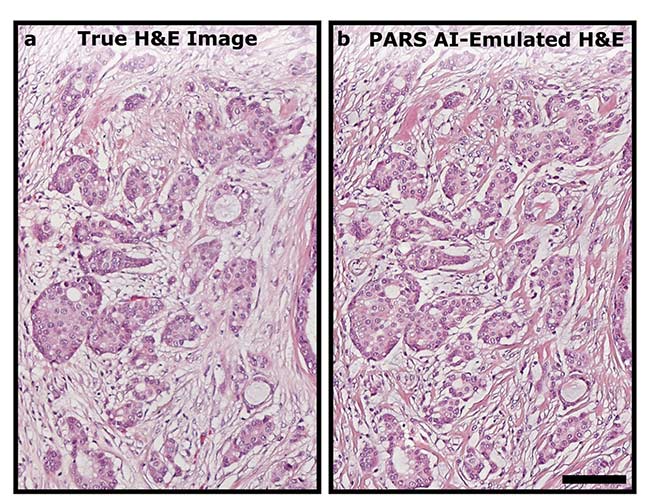
A direct one-to-one comparison between a hematoxylin and eosin (H&E)-stained slide (a) and a second-generation PARS H&E-like image (b) of the same section of human breast tissue from an unstained slide. Scale bar: 100 µm. Courtesy of PhotoMedicine Labs.
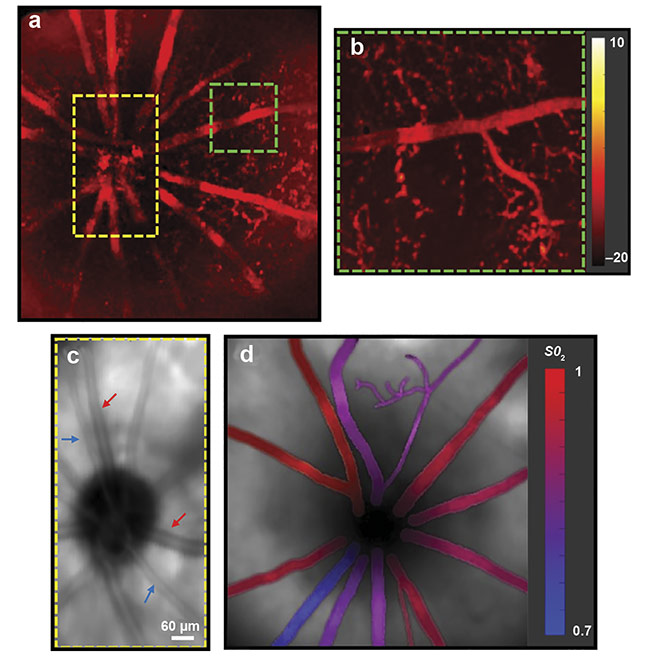
An oxygen saturation map in the retina was obtained using multiwavelength PARS imaging. A fundus PARS image acquired from large blood vessels (a). A close-up section of a blood vessel with smaller vasculature (b). A fundus image acquired using PARS (c). An oxygen saturation map in the retina obtained using multiwavelength PARS imaging (d). Courtesy of PhotoMedicine Labs.
PARS microscopy’s noncontact architecture is particularly important for ophthalmological applications, since contact with ocular tissues increases the risk of infection, abrasion, and patient discomfort. And, by applying pressure to the eye, such contact inhibits normal oxygen diffusion, skews SO2 and MRO2 measurements, and interferes with an accurate assessment of physiological and pathophysiological ocular vascular function. Additionally, contact-based technologies record involuntary eye movements, which may affect coupling efficacy and degrade image quality. Since PARS can take advantage of the various optical absorptions of different contrast agents, ongoing research and development may provide a noncontact method for measuring drug concentrations in the eye, opening a new field in pharmaceutical development and enabling pharmaceutical testing for eye diseases earlier than is currently possible.
Recently, PARS microscopy was used to image the anterior and posterior segments of the eye in rats and mice, to measure retinal pigment epithelium melanin structure, and to provide the first in vivo, noncontact photoacoustic estimation of SO2. To measure SO2, the PhotoMedicine Labs team leveraged different endogenous absorption contrasts of oxy- and deoxy-hemoglobin by using 532- and 558-nm excitation lasers, respectively, and the difference between the measurements was used to directly calculate SO2 concentrations in ocular tissues5.
The next steps in the research include developing a clinic-ready prototype using near-infrared wavelengths to reduce patient eye motion and improve imaging comfort, accuracy, safety, and repeatability, as well as developing a suite of PARS optical setups and computing algorithms to clearly and rapidly image the anterior and posterior of the eye to provide structural details, image blood vessels, obtain SO2 and MRO2 measurements, and measure blood flow in vivo. The SO2 accuracy in the eye will then be tested and validated, and second-generation PARS will be applied to take advantage of the additional optical absorption and scattering contrasts available. Throughout the research, the prototype will be tested on phantoms, on animals, and, finally, on human eyes.
Meet the author
Parsin Haji Reza, Ph.D., is a professional engineer, an award-winning teacher and researcher, an entrepreneur, a published novelist, and the inventor of photoacoustic remote sensing (PARS) microscopy. He is a co-founder of illumiSonics Inc., where he held the position of CEO from 2014 to 2018. He is currently CTO and chairman of the board of the company. He leads all scientific research and technology development, and he oversees major decisions and policies. Since April 2018, Haji Reza has been an assistant professor of biomedical engineering at the University of Waterloo; email: [email protected].
References
1. P. Hajireza et al. (2017). Non-interferometric photoacoustic remote sensing microscopy. Light Sci Appl, Vol. 6, p. e16278, www.doi.org/10.1038/lsa.2016.278.
2. P. Haji Reza et al. (2018). Deep non-contact photoacoustic initial pressure imaging. Optica, Vol. 5, pp. 814-820.
3. B.R. Ecclestone et al. (Article in preparation). Label-free complete absorption microscopy using second generation photoacoustic remote sensing. Research Square, www.doi.org/10.21203/rs.3.rs-887767/v1.
4. C.E. DeSantis et al. (2014). Cancer treatment and survivorship statistics, 2014. CA: Cancer J Clin, Vol. 64, No. 4, pp. 252-271.
5. Z. Hosseinaee et al. (2022). In-vivo functional and structural retinal imaging using multiwavelength photoacoustic remote sensing microscopy. Sci Rep, Vol. 12, No. 4562, www.doi.org/10.1038/s41598-022-08508-2.