Short light pulses from LED arrays propagate in tissue and are absorbed in blood, resulting in thermoelastic expansion that produces acoustic signals corresponding to disease characteristics.
Mithun Kuniyil Ajith Singh and Naoto Sato, CYBERDYNE Inc.
Efficient medical imaging techniques such as photoacoustic imaging help to accurately detect diseases as early as possible, resulting in timely intervention and reducing the need for medication and surgery. Conventionally used medical imaging techniques — such as ultrasound imaging, x-ray computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) — are rooted in different physics and contrast mechanisms. And their installation and operation expenses, as well as their sensitivities for various clinical applications, also differ. Beyond the limitations of these methods, an ideal medical imaging system should be able to diagnose diseases with high sensitivity and specificity at a cost that is affordable for all clinics around the world.
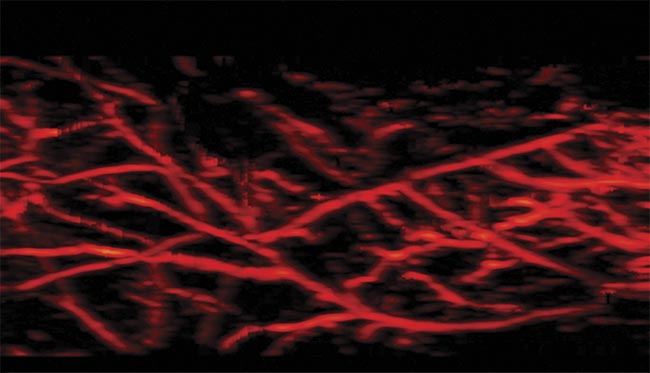
Photoacoustic imaging captures the microvasculature. Courtesy of CYBERDYNE Inc.
With its natural contrast for blood, photoacoustic imaging is one of the fastest-growing imaging modalities in both research and clinical environments. The modality has shown potential in assessing tumor vasculature, helping to manage rheumatoid arthritis by identifying where therapy is needed, and providing specific information about the causes of a plethora of inflammatory and cancer-related conditions1. In photoacoustic imaging, short light pulses (nanosecond-scale pulses in the near- to far-infrared wavelength range) are shined on tissue surfaces. This light propagates in the tissue and is absorbed by intrinsic chromophores, predominantly blood. The absorption results in ultrasound signal generation due to thermoelastic expansion. Conventional ultrasound probes placed on the surface of a patient’s skin capture these signals in real time to generate the tissue’s optical absorption map, with the concurrent resolution and imaging depth of ultrasound.
Compared to conventional optical imaging techniques, photoacoustic imaging achieves far greater imaging depths and is capable of higher resolution, making it suitable for deep-tissue, cancer-related applications, such as breast cancer imaging. Because ultrasound and photoacoustic imaging methods both rely on ultrasound signal detection, devising and implementing a dual-mode imaging
system with structural and functional contrast are straightforward tasks. Also, by tuning the wavelength of the light pulse by pulse, system users can functionally characterize tissue using photoacoustic imaging. Real-time imaging of blood oxygen saturation is useful for assessing hypoxia (low oxygen concentration in tissue), which is an important early sign of malignant cancer. Photoacoustic imaging can also detect hypervascularization (an excessive number of blood vessels) and hypoxia at early stages, making it an excellent imaging modality for detecting cancer and monitoring subsequent treatment1.
Challenges in clinical translation
The use of photoacoustic imaging has already matured in the research community, and in that setting it has demonstrated its unprecedented potential in a wide range of biomedical imaging applications. However, the translation of this promising technology into direct clinical use with patients is happening less quickly than expected2. A primary reason for the lag in implementation is that a conventional photoacoustic imaging setup requires the use of bulky, slow, and expensive pulsed lasers for tissue illumination. Large-footprint laser-based photoacoustic imaging systems also require laser-safe rooms and the use of safety goggles. Additionally, the repetition rate of such lasers is often low, resulting in low temporal resolution.
Hand-held ultrasound imaging has become common in clinics, and it would be ideal to integrate photoacoustic imaging into such systems for faster clinical translation. Integrating a bulky laser system into ultrasound machines, however, would negate portability and affordability advantages. For democratizing photoacoustic imaging and accelerating its clinical translation, clinic-friendly illumination sources must be developed.
LED arrays in photoacoustics
Fortunately, recent advancements in solid-state devices have included the development of portable, affordable, and energy-efficient LED arrays that can be used as an alternative illumination source in photoacoustic imaging. LEDs are p-n junction diodes (two-electrode semiconductor devices) that emit light when electric current passes through them. In conventional applications, LEDs are used in continuous-wave mode, while pulsed light is a prerequisite for photoacoustic imaging2. However, it is possible to operate LEDs in the pulsed mode by using higher-current pulses of lower-duty cycles (<0.1%). In this way, the optical output can be safely increased, and these LED devices can be used as an illumination source in photoacoustic imaging.
In addition to being low cost and energy-efficient, LEDs are capable of providing stable pulsed light in a wide wavelength range, from the visible to near-infrared, and at repetition rates in the kilohertz range, as opposed to pulsed lasers, which are inefficient in visible wavelengths. Several early research studies have shown that such LED elements can be used for providing pulsed light in photoacoustic imaging. However, most of these studies involved the proof-of-concept stage of experimentation, in which tissue-mimicking phantoms were used as test subjects. Human in vivo imaging was far from a reality at this point in development because of the low optical power offered by LED elements when compared to lasers. Even after overdriving, LEDs can provide optical power in the microjoule range per pulse, while lasers are capable of pumping energy in millijoule range per pulse.
In 2015, Toshitaka Agano and his colleagues demonstrated the feasibility of combining hundreds of high-power LED elements in a rectangular package and simultaneously pulsing the light sources to perform biomedical photoacoustic imaging3. Using this novel technology, Japanese company PreXion Corp. commercialized a high-power LED-array-based photoacoustic imaging system called AcousticX (Figure 1). The technology was later acquired by CYBERDYNE Inc. In this system, a single LED element of 850 nm provides an output energy of 0.024 µJ per pulse, with a pulse duration of 70 ns and a 1-A DC current. By developing LED elements with a double stack structure, arranging them in an array, and applying 20× the rated current, a light output of 200 µJ per pulse at near-infrared wavelengths is achieved. Two of these arrays are kept, one per side, on a linear array ultrasound probe for performing real-time photoacoustic and ultrasound imaging.

Figure 1. The AcousticX system (left) and various-wavelength LED arrays (right). Courtesy of CYBERDYNE Inc.
The repetition rate of the first commercial LED-based photoacoustic imaging system was 4 kHz. After its introduction, the system gained more attention and has been used by researchers around the world for various preclinical and clinical applications4.
LEDs vs. lasers
Even though LED-based photoacoustic imaging had shown convincing biomedical imaging results in research laboratories in recent years, researchers continued to question how LEDs, with their low optical output, could be used as an alternative to powerful lasers for medical purposes. No head-to-head comparison report on LED- versus laser-based photoacoustic imaging existed. This changed in 2021 when Sumit Agrawal and his colleagues from Penn State reported on a study in which they compared LED- and laser-based photoacoustic imaging, especially for vascular imaging applications5. Despite the huge difference in the optical power of LEDs compared to lasers (400 µJ vs. 40 mJ), the LED-based method performed equally well in both phantom and in vivo human volunteer experiments.
Interestingly, at certain imaging depths, LED-based photoacoustic imaging offered higher signal-to-noise ratio (SNR) than its laser-based counterpart. According to the researchers, there are several key reasons behind the better performance of LED-based photoacoustic imaging. Matching the optical pulse width with the acoustic reception bandwidth leads to efficient detection of generated photoacoustic signals. LEDs produce low noise in image acquisition, while lasers, due to high optical output, produce high background noise. And the high pulse repetition rate of LEDs offers the possibility to average more frames to improve the SNR. Figure 2 shows an in vivo comparison of LED- and laser-based photoacoustic imaging during visualization of peripheral microvasculature in a human volunteer. Results from this work demonstrate that, especially in applications involving vascular imaging, LED arrays can be an effective alternative illumination source in photoacoustic imaging.
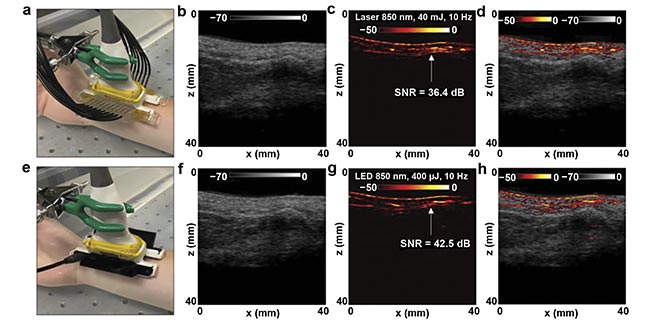
Figure 2. Comparison of LED- and laser-based photoacoustic in vivo vasculature imaging of the right-hand wrist of a healthy 25-year-old male human volunteer. The experimental setup, with the wrist placed inside a large water bath for laser-based photoacoustic imaging (a), and the images obtained via ultrasound (b), photoacoustic imaging (c), and co-registered ultrasound and photoacoustic imaging (d) for the setup shown in (a). The setup with LED arrays (e), and the images obtained via ultrasound (f), photoacoustic imaging (g), and co-registered ultrasound and photoacoustic imaging (h) for the setup shown in (e). SNR: signal-to-noise ratio. Adapted with permission from Reference 5.
In terms of energy efficiency, LEDs are far superior to solid-state lasers. With lasers, the efficiency of converting electrical power into light output is, on average, 0.1% or less. The energy conversion efficiency of pulsed LEDs, however, is about 6%, making them an ideal low-power choice for portable photoacoustic imaging equipment.
3D visuals of microvasculature
Imaging superficial and subsurface microvasculature with good spatial resolution (see image on page 39) is important for diagnosing disorders such as skin cancer and peripheral vascular diseases, as well as for monitoring treatment. Even though larger blood vessels can be detected using techniques such as ultrasound and MRI, inadequate spatial resolution impedes the accurate assessment of microvasculature. When using LEDs as an illumination source in photoacoustic imaging, apart from real-time 2D imaging, it is feasible to scan the probe linearly over the subject to generate high-resolution 3D maps of vasculature6. Figure 3 shows imaging locations on the foot of a human volunteer. 3D LED-based photoacoustic imaging, combined with ultrasound, demonstrates potential in assessing microvascular health and could become an affordable point-of-care tool for effective diagnosis and treatment.
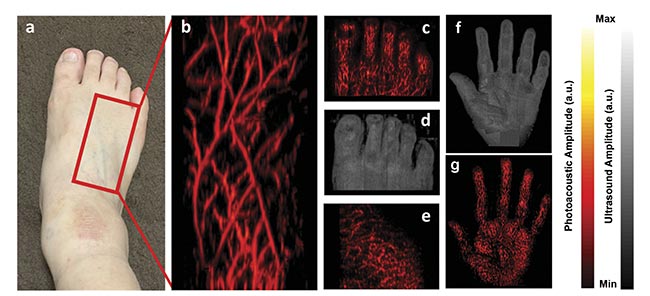
Figure 3. The location on a human foot where the imaging was performed (a). A 3D maximum intensity projection (MIP) photoacoustic image of the area marked in (a), visualizing fine microvasculature network (resolution: ~200 μm) (b). A 3D MIP photoacoustic image of the foot’s toes (c). A 3D MIP ultrasound image of the toes (d). A 3D MIP photoacoustic image of the plantar area of the foot (e). A 3D structural ultrasound MIP image of the palm of a human hand, generated by combining 10 line-by-line scan ultrasound MIP images (f). A 3D vascular photoacoustic MIP image of the palm generated by combining 10 LED-based photoacoustic MIP images (g). Courtesy of CYBERDYNE Inc.
Oxygen saturation imaging
Most tumors are heterogeneous in oxygen saturation, and they develop regions with hypoxic cells during growth. It is well known that cancers with extensive hypoxic regions are resistant to radiation and chemotherapy, and that tumor hypoxia may promote malignant progression and metastatic dissemination. Since hypervascularization and reduction in oxygen saturation may happen at an early stage of malignancy development, hypoxia is indeed an early marker for cancer detection. And because oxygenated and deoxygenated hemoglobin have defined optical absorption spectra, using dual-wavelength photoacoustic imaging to estimate the oxygen saturation with high resolution is a straightforward task. Such imaging can estimate oxygen at the microvasculature level, while conventional pulse oximeters estimate only average oxygen saturation values.
Figure 4 shows the results of the proof-of-concept work in which two-wavelength (750 and 850 nm) LED-based photoacoustic imaging was used to image oxygen saturation of microvasculature in a human volunteer’s wrist7. In a healthy human, oxygen saturation in a vein compared to an artery differs by a maximum of 10% to 15%. And it is noteworthy that the LED-based imaging method could differentiate venous and arterial blood with high spatial resolution (~200 μm) and high temporal resolution (or 15 Hz with combined ultrasound and photoacoustic imaging). Thus, the combined imaging of hypervascularization and hypoxia in tumors using LED-based photoacoustic imaging has excellent potential for use in cancer diagnosis and treatment planning.
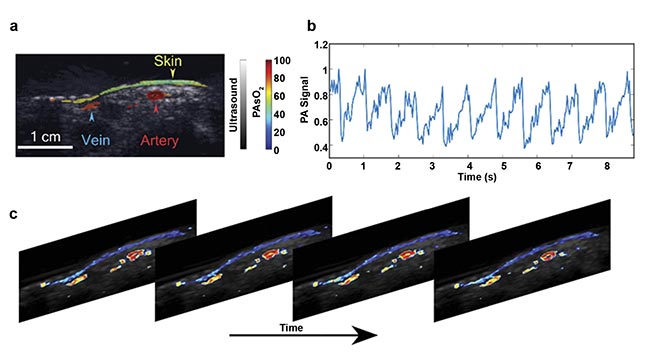
Figure 4. In vivo imaging of the oxygen saturation of blood vessels in a human wrist, acquired using dual-wavelength (750 and 850 nm) LED-based photoacoustic imaging. An image of fluence-compensated photoacoustic (PA)-based oxygen saturation (sO2) of a human wrist overlaid on an ultrasound image (a). The normalized photoacoustic signal from an artery showing pulsation (b). PA sO2 frames showing the pulsating artery (c). Adapted with permission from Reference 7.
Future outlook
Because of LED arrays’ portability, affordability, energy efficiency, and pulse-width tunability (the light pulse width can be matched with the ultrasound probe reception bandwidth) — as well as the availability of wide optical wavelength range — LEDs can be used as an effective alternative to lasers in biomedical photoacoustic imaging. The addition of LED-based photoacoustic imaging into conventional pulse-echo ultrasound imaging in a clinical-grade machine could have a profound impact on point-of-care diagnostic imaging, and it could also accelerate the clinical translation of photoacoustics, especially in cancer imaging applications.
LEDs and their drivers are widely used in consumer electronics, and, so, medical applications could also benefit from the economies of scale and cost reductions that arise from high-volume manufacturing. LED-based photoacoustic imaging undoubtedly has strong potential for use in point-of-care noninvasive cancer imaging applications to complement clinical ultrasound imaging. With the quickly evolving developments in semiconductor technology that are enabling miniaturization, and with artificial intelligence helping to swiftly gather data and accelerate diagnoses, the LED-based imaging method will likely translate from the bench to bedside in the near future.
Portable and low-cost imaging devices that provide deep-tissue structural, functional, and molecular contrast with high spatial and temporal resolution may have a great impact. Even though diverse hurdles and challenges exist for the clinical translation of LED-based photoacoustic imaging, the technique shows great potential to revolutionize the field of medical imaging.
Meet the authors
Mithun Kuniyil Ajith Singh is an engineering scientist with extensive experience in preclinical and clinical photoacoustic and ultrasound imaging. He is currently the research and business development manager at CYBERDYNE Inc. in the Netherlands, where he initiates and coordinates various scientific projects in collaboration with globally renowned research groups, focusing especially on the clinical translation of LED-based photoacoustic imaging technology. Singh earned a doctorate in 2016 from University of Twente under the supervision of professor Wiendelt Steenbergen. While working on his doctorate, he invented
a method called PAFUSion (photoacoustic-guided focused ultrasound) for reducing reflection artifacts and improving contrast and imaging depth in photoacoustic imaging; email: [email protected].
Naoto Sato is a research manager with 32 years of combined engineering and management experience in the field of biomedical imaging (photoacoustic and ultrasound imaging). He is the R&D specialist at CYBERDYNE Inc. in Japan, leading the development of LED-based photoacoustic and ultrasound imaging technology. Previously, he worked at GE Healthcare in the ultrasound imaging division, from 1987 to 2013, where he was a key player involved with the technical and management aspects of several ultrasound imaging system development projects; email: [email protected].
References
1. L. Lin and L.V. Wang (2022). The emerging role of photoacoustic imaging in clinical oncology. Nat Rev Clin Oncol, Vol. 19,
pp. 365-384.
2. M. Kuniyil Ajith Singh (2020). LED-Based Photoacoustic Imaging. Springer Nature, Singapore Pte. Ltd.
3. T. Agano et al. (2015). Comparative experiments of photoacoustic system using laser light source and LED array light source. Proc SPIE, Vol. 9323: Photons Plus Ultrasound: Imaging and Sensing, San Francisco, p. 93233X.
4. Y. Zhu et al. (2020). Towards clinical translation of LED-based photoacoustic imaging: a review. Sensors, Vol. 20, p. 2484.
5. S. Agrawal et al. (2021). Photoacoustic imaging of human vasculature using LED versus laser illumination: a comparison study on tissue phantoms and in vivo
humans. Sensors, Vol. 21, p. 424.
6. M. Kuniyil Ajith Singh et al. (2022).
Three-dimensional handheld LED-based photoacoustic/ultrasound imaging: a potential point-of-care tool for diagnosing peripheral arterial disease. Proc SPIE,
Vol. 11960: Photons Plus Ultrasound:
Imaging and Sensing.
7. R. Bulsink et al. (2021). Oxygen saturation imaging using LED-based photoacoustic system. Sensors, Vol. 21, p. 283.