When creating a new technology – or tweaking an old one – you always end up starting from the bottom and working your way up. No, not your position in the company, the basic nature of the materials with which you are working.
It doesn’t matter whether you are looking to make a high-efficiency solar collector, artificial neurons or a better mousetrap, you must have as complete an understanding of the components as possible. Once, that meant having only a good knowledge of macro materials; now, you need to know what’s going on at the molecular level.
For the most part, materials research at this level involves shunting nanoclusters of molecules, such as cadmium selenide, or of individual elements, such as silver, around a flat substrate. Types of this research include quantifying the electro-optical properties of carbon nanotubes and quantum dots.
More recently, scientists have become interested in how particles of various sorts operate in aqueous environments, such as living cells or solutions in which nanoparticles may be formed or other chemical reactions may be induced. Tracking minute particles in solution (even, in some cases, as they are precipitating out) can provide important clues to the dynamics under-pinning the device in which the particles ultimately may be used.
Breaking down
One set of researchers recently reported that a typical method for preparing samples for inductively coupled plasma optical emission spectroscopy (ICP-OES) has room for improvement. In ICP-OES – the gold standard for studying minute particles in liquid environments – a relatively large material sample is broken down into tinier and tinier bits. These particles, sometimes as small as single molecules, are then scanned by a probing laser beam for spectroscopic analysis.
A new direction, developed by E.V. Muravitskaya and her colleagues at B.I. Stepanov Institute of Physics in Minsk, Belarus, uses such a laser beam more directly. Because acids or other substances used to break down a sample typically do their jobs incompletely, the scientists used a 1064-nm Q-switched Nd:YAG laser to ablate their target samples.
They tested their system on several alloys that were alike in composition – comprising zinc, iron, aluminum, copper and magnesium – but differing in concentration of these elements. They placed samples of the alloys into distilled water and used the laser to irradiate them with 10- to 12-ns pulses at 10 Hz (8 μs between pulses). Each pulse provided 50 mJ of energy at the sample surface. After 3 s, they moved the sample to ablate a different part. After about 80 s, ablation was complete, with the original samples left pitted and cratered and nanometer-scale particles clouding the solution. They compared their laser ablation results with a standard chemical digestion method as well.
The investigators noted in the February issue of Spectrochimica Acta Part B that using laser ablation to divide large particles into a size fit for ICP-OES has several advantages. First, compared with chemical erosion, laser ablation does not waste sample material because it more completely breaks down the mass. Second, ablation does not cause the suppressive effect that acids have on signals picked up by the spectrographic equipment. Furthermore, laser ablation produces more uniform particle sizes and apparently works with materials that are not greatly affected by typical acids used in chemical erosion techniques.
Another solution
ICP-OES is not the only form of spectroscopy performed on materials in solution. Once relegated to testing particles splayed out on wide-open metal substrates, Raman spectroscopy now also is being used to explore substances in aqueous environments. In traditional surface-enhanced Raman spectroscopy (SERS), investigators use a silver or gold film to enhance the otherwise very weak Raman signal that is emitted by a test material. Performing SERS on a flat substrate is one thing, but using it to study particles in cells or other aqueous solutions requires getting the metal particles into place and understanding how they will act.
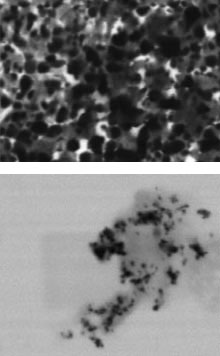
Images acquired with a JEOL Ltd. scanning transmission electron microscope show silver nanoparticles with an average size of 15 nm (bottom) and 45 nm (top). The smaller particles provide greater enhancement of the Raman signal in SERS experiments. Courtesy of the Journal of Physical Chemistry C.
A number of researchers are starting to extend the utility of SERS-like signal enhancement by inverting the relationship between the test subject and the substrate.
Creating metal nanoparticles and placing them near the particles of interest in their liquid home environment provides as much Raman signal enhancement as using a metallic substrate. This knowledge, however, was not enough for Caryn S. Seney and Brittany M. Gutzman of Mercer University in Macon, Ga., and their colleague, Russell H. Goddard of Valdosta State University, also in Georgia. They wanted to know whether the size of metallic nanoparticles had an influence on the Raman enhancement effect.
The group created silver nanoparticles ranging from 15 to 160 nm across, placed them in solution with BPE, a standard compound used in Raman studies of solutions, and applied an argon-ion laser made by Coherent Inc. to excite them. As the scientists reported in the Jan. 8 issue of the Journal of Physical Chemistry C, the Raman effect is indeed tied to the size of the silver particles.
According to the researchers, SERS activity generally is inversely proportional to particle size – the smaller the particle, the higher the Raman signal. In their study, they found that the 15-nm silver particles had the highest signal enhancement, and that the highest effective laser wavelength to obtain the SERS signal was 390 nm.