Nanotechnology-based imaging offers accurate, real-time feedback in the operating room, enabling greater precision in the removal of the entire malignant tumor to prevent recurrence.
RALUCA BORLAN, MONICA FOCsAN, AND SIMION AsTILEAN, BABEs-BOLYAI UNIVERSITY; AND PATRICIU ACHIMAs-CADARIU, THE ONCOLOGY INSTITUTE ‘PROF. DR. ION CHIRICUTA’
Despite discoveries made in cancer research in recent years, few new techniques have significantly changed the course of the disease, which is responsible for more than 25,000 deaths in the U.S. every day1. Surgery is usually the most effective treatment for cancer, in combination with perioperative systemic therapy in selected cases. Despite the efficacy of this course, the disease can reappear2. If the pathological report describes positive reactive margins in the area where surgery occurred, a second intervention may do more harm than good. Depending on the patient’s postoperative status, he or she may not be able to tolerate a second significant surgery. But near-infrared (NIR) fluorescence can help to negate the need for more surgery.
Cancer cells in the human body. Courtesy of iStock.com/wildpixel.
None of the classical methods used in cancer detection and surgical planning can be readily implemented in an operating room, due to the required positioning of the patient and to technical constraints. These methods include computed tomography, single-photon emission computed tomography, scintigraphy, positron emission tomography, and magnetic resonance imaging. Specific strict safety measures must be followed by medical personnel when they work with ionizing radiation. Computed tomography is limited when describing lesions smaller than 10 mm, despite its high-resolution whole-body imaging capabilities. Even the highly trained eyes of surgeons can only detect tumors larger than 50 µm in ideal conditions. These restrictions make it very hard to discern between healthy and malignant tissue in patients with small peritoneal metastases.
Nanotechnology offers promise in medicine and, particularly, in cancer treatment by providing real-time feedback that the classical pre-operative imaging techniques cannot provide to surgical oncologists during an operation. In recent years, state-of-the-art NIR fluorophores encapsulated in nanoparticles have gained popularity in their use as fluorescent probes and contrast agents for the imaging of cancer surgery while it is being performed. Using fluorophores with emission in the NIR region of the spectrum (700 to 1200 nm), also known as the biological spectral window, provides clear advantages for imaging tumors during surgery, due to NIR light’s low autofluorescence (relatively little background noise) and deep tissue penetration.
Unlike free fluorophores, which can aggregate in a physiological environment, fluorophore-encapsulated nanoparticles with diameters in the optimal size range circulate in the blood for an extended period of time. Because of their high loading capacity and protective properties, they suppress the poor photostability of free NIR dyes. Moreover, depending on the molecules’ therapeutic features, the photothermal and photodynamic performance of the fluorophore can be improved after encapsulation within nanoparticles. Therefore, novel advancements in the fabrication and implementation of tailored nanoparticles could readily translate to clinical settings for visualizing and resecting tumors, and could even assist in the cancer treatment itself.
NIR vs. classical imaging
By adding fluorescence imaging to their standard techniques, surgeons can benefit from its high-resolution images because of its ability to detect lesions smaller than 10 µm, its relatively low costs, and the easy translation of the fluorescence microscope to the operating room. By integrating intraoperative fluorescence systems within surgical microscopes (Figure 1), surgeons benefit from using an optical system that is capable of recording and overlapping two imaging channels, thereby attaining uninterrupted work flow during cancer resection and enhanced visualization and detection of tumor margins. Although fluorescence imaging combined with the administration of contrast agents is currently a prevalent method used for the delineation of diseased tissue, most clinical applications are limited to the use of fluorescence agents that emit light in the visible range of the electromagnetic spectrum.
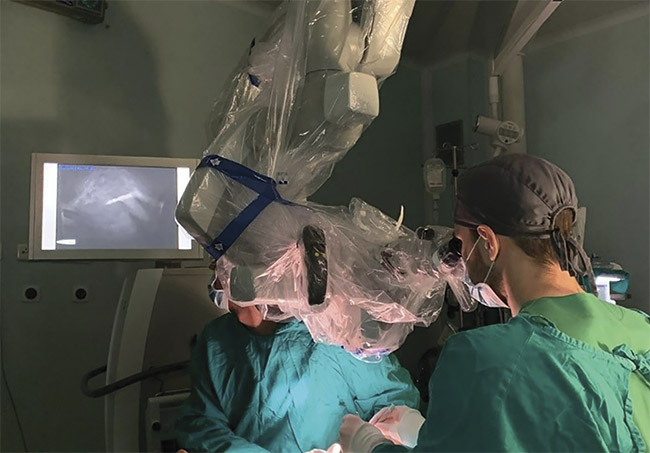
Figure 1. An experimental in vivo design of a surgical NIR fluorescence microscope, demonstrating the feasibility of a new intraoperative imaging technique that specifically targets tumor areas to improve surgical outcomes and patient quality of life. Courtesy of The Oncology Institute ‘Prof. Dr. Ion Chiricuta.’
And the results don’t always provide straightforward information. Because hemoglobin, proteins, and other components of the blood have higher absorbance coefficients in the 200- to 650-nm region of the spectrum and above 1450 nm, a major flaw in the use of contrast agents with optical features in the ultraviolet-visible range is the enhanced autofluorescence, absorption, and scattering of light by the biological tissue. To combat the shortcomings of fluorescence imaging in the visible domain, as well as those of other classical imaging techniques, NIR fluorescence imaging is now being investigated to attain deep tissue penetration (up to 2 cm). Its use could also offer enhanced precision of tumor resection and real-time feedback during surgery, and, most importantly, improve the quality of life for cancer patients (Figure 2).
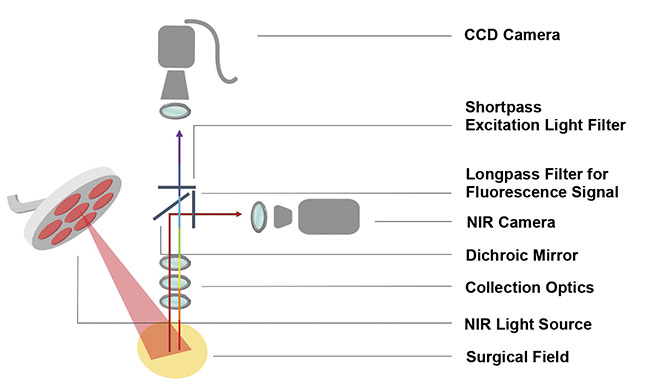
Figure 2. A schematic representation of an NIR fluorescence surgical microscope. Courtesy
of Raluca Borlan.
In vivo assay in murine models
Today, organic fluorophores remain the cornerstone of NIR contrast agents for fluorescence imaging. However, due to the fluorophores’ low fluorescence stability, poor solubility in water, and tendency to form clusters, the research community is seeking alternatives to using freeform fluorophores in the standard administration of medication into the circulatory system. Thus, to enhance and maintain the NIR fluorophores’ utility for a prolonged period of time during tumor molecular imaging, an evolving technique is to encapsulate them within personalized, biocompatible nanocarriers. These nanocarriers (nanoagents/nanoparticles) are fabricated from organic compounds (for example, lipids, proteins, carbohydrates, and noncytotoxic synthetic materials) that will not cause a toxic effect to living tissue and are functionalized to target specific cancer markers individualized to patient needs.
One of the main advantages of implementing NIR fluorescent nanoparticles versus free fluorophores as optical contrast agents in interventional imaging of cancer is the nanoparticles’ accumulation at a tumor site. This phenomenon is achieved through the active targeting of specific cancer markers by decorating the nanoparticles with appropriate antibodies or other ligands, or through passive tumor targeting via the enhanced permeability and retention effect. This effect results in the tendency of molecules or nanoagents — for example, nanoparticles, liposomes, or macromolecular drugs with sizes between 6 and 300 nm — to accumulate nonspecifically and remain in the cancerous tissue due to low lymphatic drainage. The effect takes place because of the rapid development of new blood vessels around the tumor, resulting in the accelerated development and hypervascularity of the malignant tissue, a key component in the development of cancer.
Treating glioblastoma
In a recent study, mice injected with human primary glioblastoma cells were investigated after administration of an
FDA-approved NIR fluorophore, indocyanine green, in its free form and encapsulated within protein nanoparticles (Figure 3). NIR fluorescent nanoparticles enhanced the free fluorophore’s penetration of the malignant tissue by a 38-fold increase in fluorescence intensity at the tumor site.
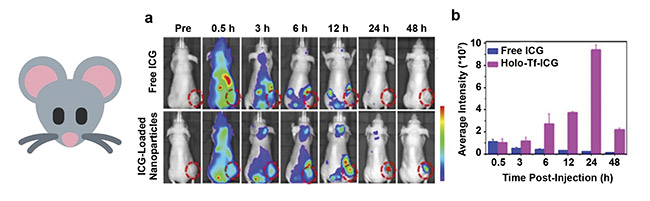
Figure 3. In vivo fluorescence imaging of nude mice subcutaneously injected with human primary glioblastoma cells (U87MG cell line) after intravenous
administration of free indocyanine green (ICG) and transferrin nanocarriers loaded with ICG. Tumor tissue is delineated with a dashed line (a). A quantitative assay of the fluorescence intensity within the cancerous tissue with respect to post-administration time (b). Adapted with permission from Reference 3/American Chemical Society.
The enhanced capabilities of the same indocyanine green, after encapsulation within human serum albumin nanocarriers, were conclusively proven by ex vivo investigations after tumor and organ dissection of mice bearing human breast cancer cell xenografts. Imaging at 24 h after administration of the NIR nanoparticles showed good tumor penetration and high fluorescence intensity recorded for indocyanine green-loaded protein nanocarriers. This compared favorably to the free form of the fluorophore, which accumulated mostly in the liver, and only dim fluorescence signals could be observed at the tumor site (Figure 4).
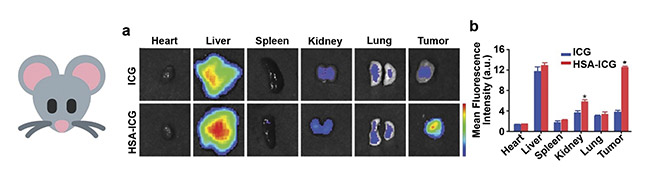
Figure 4. Ex vivo fluorescence imaging of nude mice bearing human breast cancer cell xenografts (MDA-MB-231-luc cell line), after intravenous administration of free ICG and human serum albumin (HSA) nanocarriers loaded with ICG (a). A semiquantitative assay of the fluorescence intensity measured for the same samples (b). Adapted with permission from Reference 4/Frontiers in Oncology.
Human clinical trials
To achieve better post-operative outcomes and considerable quality-of-life improvements for cancer patients, a new generation of contrast agents for the real-time NIR fluorescence imaging of malignant tissue during cancer surgery is greatly needed. However, although pre-clinical studies have demonstrated successful outcomes using nanosize NIR fluorescent contrast agents for real-time imaging of cancerous tissue, the predominant optical agents approved by the FDA for human use are still free fluorophores with optical features in the visible region of the electromagnetic spectrum.
One of the few classes of nanocarriers that is currently implemented in clinical trials is silica-based nanoparticles. As depicted in Figure 5, an NIR fluorescence-emitting dye (Cy5.5) was loaded within silica nanoparticles to aid in the detection of the sentinel lymph node in the parotid gland of a male patient with melanoma. The nanoparticles provided promising information regarding biological and physicochemical features for the interventional NIR fluorescence imaging of cancer. The study concluded that no toxic effect was observed from using NIR nanoparticles — confirming a good consonance rate between fluorescence and technetium (Tc)-99m detection of the sentinel lymph nodes — while also proving that the procedure is reliable and clinically viable.
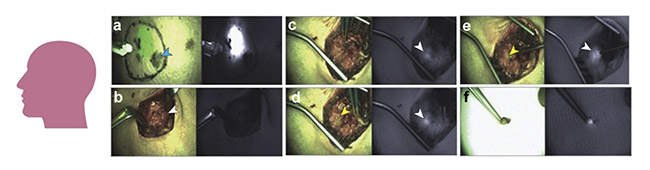
Figure 5. Composite (green signal) and adjacent fluorescence (white signal) displays of a male patient injected with Cy5.5-loaded silica nanoparticles near a scalp melanoma (a). The progressive increase in fluorescence signal corresponding to a metastatic intraparotid 1.5- to 2-cm-deep sentinel lymph node (b-e). Ex vivo sentinel lymph node (f). Adapted with permission from Reference 5/JAMA Network Open.
Tailored nanoparticles that are loaded with NIR fluorophores and functionalized with specific cancer ligands have been shown to be an effective approach for the real-time NIR fluorescence imaging of tumors during oncologic surgery. These nanoparticles are clearly ready for the next research step — clinical trials involving human patients.
Meet the authors
Raluca Borlan is in the final year of her doctoral studies in the Faculty of Physics at Babes-Bolyai University in Cluj-Napoca, Romania. Her ongoing research interest is focused on the development and characterization of organic nanoparticles loaded with near-infrared fluorophores as contrast agents for real-time image-guided cancer surgery; email: [email protected].
Monica Focsan, Ph.D., is senior researcher at Babes-Bolyai University. She received her doctorate in physics in co-direction from Joseph Fourier University/Babes-Bolyai University. Her current interests involve interdisciplinary research focused on nanobiophotonics, with an emphasis on the synthesis and characterization of various versatile nanoparticles with particular morphologies for molecular biosensing, bioimaging, and therapeutic applications; email: [email protected].
Simion Astilean is a professor in the Faculty of Physics and director of the Nanobiophotonics and Laser Microspectroscopy Centre at Babes-Bolyai University. His research group (www.nanobiophotonics.ro) specializes in the synthesis of multifunctional hybrid nanoplatforms for applications in nanomedicine, biosensing, cell imaging, therapy, and drug delivery. His current research is focused on developing new targeted optical imaging nanoprobes for NIR real-time image-guided surgery of ovarian cancer; email: [email protected].
Patriciu Achimas-Cadariu is a professor at the Iuliu Hatieganu University of Medicine and Pharmacy in the Department of Surgical and Gynecologic Oncology, and a consultant physician at The Oncology Institute “Prof. Dr. Ion Chiricuta,” both in Cluj-Napoca, Romania. Achimas-Cadariu is a member of the European Society of Gynecological Oncology. His research is focused in large part on ovarian cancer surgery and the evolution of and prognostic factors in ovarian cancer, quality-of-life assessment in oncology, and cancer in pregnancy; email: [email protected].
References
1. WebMD (May 2020). How many people die of cancer a year? www.webmd.com/cancer/how-many-cancer-deaths-per-year.
2. R.R. Zhang et al. (2017). Beyond the margins: real-time detection of cancer using targeted fluorophores. Nat Rev Clin Oncol, Vol. 14, Issue 6, pp. 347-364, www.doi.org/10.1038/nrclinonc.2016.212.
3. M. Zhu et al. (2017). Indocyanine green-
holo-transferrin nanoassemblies for tumor-targeted dual-modal imaging and photothermal therapy of glioma. ACS Appl Mater Interfaces, Vol. 9, Issue 45, pp. 39249-39258, www.doi.org/10.1021/acsami.7b14076.
4. Z. Wang et al. (2021). Human serum albumin decorated indocyanine green improves fluorescence-guided resection of residual lesions of breast cancer in mice. Front Oncol, Vol. 11, p. 614050, www.doi.org/10.3389/fonc.2021.614050.
5. D.K. Zanoni et al. (2021). Use of ultrasmall core-shell fluorescent silica nanoparticles for image-guided sentinel lymph node biopsy in head and neck melanoma: a nonrandomized clinical trial. JAMA Netw Open, Vol. 4, Issue 3, p. e211936, www.doi.org/10.1001/jamanetworkopen.2021.1936.