PASADENA, Calif., April 14, 2022 — Researchers at the University of Southern California (USC) developed a printing technique that could provide the precision required to successfully 3D-print microfluidic channels on chips at a scale not previously seen. The technique is called in situ transfer vat photopolymerization (IsT-VPP).
The use of microfluidic devices — compact testing tools composed of tiny channels carved on a chip — can lower the cost of drug development and aid in medical diagnostics. The traditional approach to fabricating these devices, soft lithography in a cleanroom setting, requires multiple labor-intensive processes.
While 3D printing offers many advantages for biomedical device manufacturing, it is not yet sensitive enough to build layers with the minute detail required for microfluidics.
Using the IsT-VPP technique, the researchers were able to produce microfluidic channels 10 μm in height and of high accuracy (within the 2-μm level), without use of a liquid resin with reduced transparency or decreased fabrication speed. Vat photopolymerization (VPP) uses liquid photopolymer resin to build the item to be printed layer by layer. The item is then irradiated with ultraviolet (UV) light, which cures and hardens the resin at each layer. A build platform moves the printed item up or down so additional layers can be built onto it.
Although VPP allows for one-step fabrication, its control of the microfluidic device’s micron-size channels falls short. The UV light source has a tendency to penetrate deep into the residual liquid resin, curing and solidifying material within the walls of the channels.
“When you project the light, ideally, you only want to cure one layer of the channel wall and leave the liquid resin inside the channel untouched, but it’s hard to control the curing depth, as we are trying to target something that is only a 10-μm gap,” professor Yong Chen said.
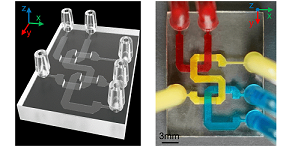
An example of a microfluidic chip created by the USC research team The researchers’ method of microfluidic device fabrication is compatible with commonly used 405-nm light sources and commercial photocurable resins. Courtesy of Yang Xu.
While opaque resin allows less light penetration than transparent resin, it is not suitable for building a microfluidic device whose contents will be examined under a microscope.
To create channels in clear resin at the microscale needed for microfluidic devices, the team developed an auxiliary platform that moves between the light source and the printed device, blocking the light from solidifying the liquid within the walls of a channel.
When the channel roof (i.e., the top layer portion of the device that encloses the channel) is printed, the auxiliary platform is used to prevent the light from penetrating into the residual liquid resin inside the channel. The channel roof is then in situ transferred to the built part. All the other layers are printed using the standard VPP process. Any residual resin in the channel remains in a liquid state and can be flushed out after the printing process to form the channel space.
According to Chen, current commercial processes only allow for the creation of a microfluidic channel height at the 100-µm level and provide poor control over accuracy.
“This is the first time we’ve been able to print something where the channel height is at the 10-µm level,” he said. “We can control it really accurately, to an error of plus or minus 1 µm. This is something that has never been done before, so this is a breakthrough in the 3D printing of small channels.”
The USC VPP-based technique is compatible with commonly used 405-nm light sources and commercial photocurable resins. The researchers verified the technique by fabricating multifunctional devices, including 3D serpentine microfluidic channels, microfluidic valves, and particle sorting devices.
Chen said that the new 3D-printing platform, with its microscale channels, could offer significant benefits to cancer detection and research.
“Tumor cells are slightly bigger than normal cells, which are around 20 µm. Tumor cells could be over 100 µm,” he said. “Right now, we use biopsies to check for cancer cells, cutting organ or tissue from a patient to reveal a mix of healthy cells and tumor cells. Instead, we could use simple microfluidic devices to flow the sample through channels with accurately printed heights to separate cells into different sizes so we don’t allow those healthy cells to interfere with our detection.”
The IsT-VPP technique for microfluidic device fabrication could advance VPP’s use for 3D-printing devices for applications requiring small gaps with high accuracy.
“There are so many applications for microfluidic channels,” Chen said. “You can flow a blood sample through the channel, mixing it with other chemicals so you can, for example, detect whether you have COVID or high blood sugar levels.”
The research team is filing a patent application for the 3D-printing method, and it is seeking collaborators to commercialize the fabrication technique for medical testing devices.
The research was published in Nature Communications (www.doi.org/10.1038/s41467-022-28579-z).