Researchers from Washington University in St. Louis have found a mechanism to efficiently and accurately measure the point spread functions (PSFs) of the position of molecules, called variance upper bound (VUB). They believe this will someday aid scientists and clinicians as they look to understand what is happening at the molecular level in the body.
The researchers specifically used fluorescent molecules to bind with amyloid fibers and measure their differences in structure. As it turns out, in so doing, they found a way to bypass typical limitations associated with single-molecule localization microscopy (SMLM) and single-molecule orientation localization microscopy (SMOLM) in the laboratory, which have difficulty tracking molecular movement.
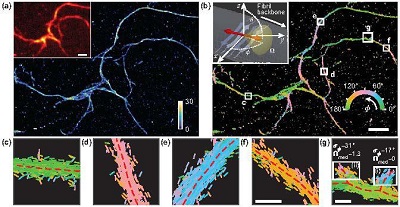
Transient amyloid binding (TAB) SMLM and SMOLM using Nile red (NR). (a) SMLM image of a network of Aβ42 fibrils. Color bar: localizations per bin (20 × 20 nm2). Inset: diffraction-limited image. (b) TAB SMOLM image, color-coded according to the mean azimuthal (ø) orientation of NR molecules within each bin. Inset: main binding mode of NR to β-sheets, that is, dipole moments aligned mostly parallel to the long axis of a fibril (its backbone). (c-g) All individual orientation measurements localized along with fibril backbones within the white boxes in (b). Scale bars: (a, b) 1 µm, (f, g) 100 nm. Courtesy of Matthew Lew.
“SMOLM is useful for seeing how molecules are assembled or how they move in a variety of soft matter, that is, squishy, liquid-like system,” said Matthew Lew, an assistant professor in the Preston M. Green Department of Electrical & Systems Engineering at Washington University. “For example, SMOLM can be used to see how molecular motors move cargo inside cells, how receptors open and close to let molecules in and out of a cell, or to see how molecules move within the pores of a nanoparticle for alternative energy applications.”
A recently published study from Lew and his colleagues pointed out that while PSFs measure only one orientation at a time, the method they devised can measure many at one time, and swiftly. They reported that with the aid of VUB, the team was able to measure fluorescence attached to amyloid fibers and accurately image differences in these networks.
The team used Nile red (NR), a fluorescent molecule that binds with a biological structure and emits light only when it is attached to a particular target of study.
Using VUB, the researchers said they were able to enhance the effectiveness of SMOLM in measuring the positioning of fibrils commonly seen in the brains of those with Alzheimer’s and Parkinson’s diseases. They observed the binding of the fluorescent molecules along the long axis of the fibrils.
“All fibrils look the same under a normal fluorescence microscope, but SMOLM sees differences in how these fibrils formed,” Lew said.
The researchers envision transient amyloid binding SMOLM providing vital clues in structure and toxicity that would aid the search for effective treatment of these conditions.
“The drug industry has spent tens of billions of dollars making drugs that are very effective at removing all systemic amyloid beta from patients, with no clinical benefit,” Lew said. “The structures of these protein assemblies are correlated with the positive and detrimental effects they have on neurons, but this exact relationship is nebulous and not well understood. We are using SMOLM to push the frontiers of this understanding.”
This research was published in Optica (www.doi.org/10.1364/OPTICA.388157).