JOSE POZO AND ANA BELÉN GONZÁLEZ GUERRERO, EUROPEAN PHOTONICS INDUSTRY CONSORTIUM
Because lasers can offer less-invasive procedures for both diagnosis and treatment, their role in the medical field is on the rise. Lasers can be modulated in wavelength, power, density, and energy, and they have a wide range of applications in ophthalmology, lithotripsy, and oncology as well as in dermatologic and cosmetic procedures (Figure 1a).
With slight modifications of the system, the same basic principles could be applied to a vast variety of tissue types. The effect a laser has on a particular tissue type is as dependent on the properties of the laser as on the properties of the tissue (Figure 1b). Body tissue varies in its water content, density, structure, thermal conductivity, and heat capacity, and these properties determine its interaction with light, which provides valuable information when a tissue is affected.
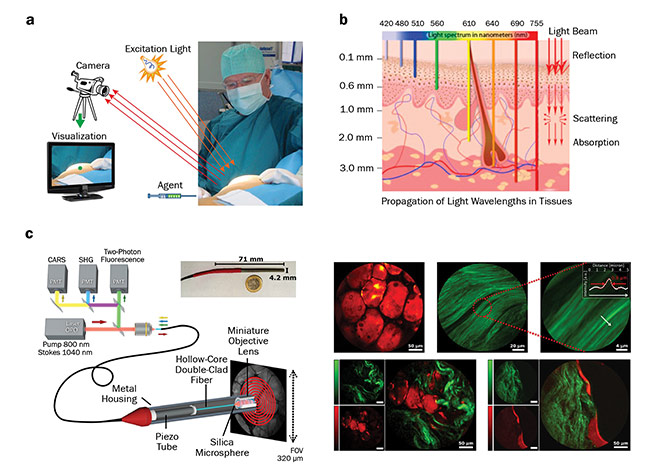
Figure 1. Use of optical imaging during surgery. The imaging system requires a light source, a detector or a camera, and a visualization system.
A special agent is applied prior to imaging (a). Penetration depth of different wavelengths of light into the skin, and the light interactions within the tissue (b). Schematic of a multimode imaging system integrated in an endoscopic device (c, left); false-colored images of the human tissue, with fat highlighted in red and collagen in green (c, right). PMT: photomultiplier tube; CARS: coherent anti-Stokes Raman scattering; SHG: second-harmonic generation; OPO: optical parametric oscillator. Image a: courtesy of Leiden University Medical Center; b: courtesy of the University of Liège; c: adapted from Light: Science & Applications, https://doi.org/10.1038/s41377-018-0003-3.
The rapid development of light sources — such as miniaturized quantum cascade lasers (QCLs) for the mid-IR region — as well as of detection infrastructure have made it possible to offer clinics numerous complex new techniques, methods, and noninvasive tools for diagnosis and surgery.
Medical imaging
Medical imaging is used to create a visual representation of the body for clinical analysis, diagnosis, and medical intervention. By revealing internal structures, it allows the practitioner to identify abnormalities.
Conventional medical imaging includes techniques such as x-ray, MRI, ultrasonography, and endoscopy, each of which provides different information about the area of the body being studied or treated, relating to possible disease, injury, or the effectiveness of a medical treatment. While these techniques are well-established and widely used, new noninvasive medical imaging techniques are needed that could provide more information regarding morphology, structure, metabolism, and function. Studies conducted in research institutions such as Leiden University Medical Center (LUMC), the Leibniz Institute of Photonic Technology, the Netherlands Cancer Institute (NKI), the Swiss Center for Electronics and Microtechnology (CSEM), and the Tyndall National Institute (TNI) demonstrate the potential and added value of new biophotonics-based techniques. Further developments in lasers, fibers, microlenses, filters, and other elements, as well as in fully integrated systems, bring biophotonics to the medical field as a supporting tool during diagnosis, surgery, and other decision-making steps in clinical practice.
Spectroscopy
Raman spectroscopy can provide detailed information about the chemical composition of tissue at high spatial resolution. It is nondestructive and label-free, and can be easily combined with other methods. Coherent Raman scattering (CRS) can add detailed morphological information and images based on individual vibrational levels. While the advantages and capacity of Raman technologies for single-cell and tissue analysis are impressive, the need for a trained scientist for data acquisition and processing can hinder the real application of these systems in a clinical environment.
At the same time, the requirements for wavelength stability and accuracy, cleanliness, and robustness of the spectrum can be fulfilled with laser sources provided by companies such as Cobolt AB. For CRS, one laser source is not sufficient. A tunable source is also required, and the two sources must be perfectly aligned in time and space. Angewandte Physik & Elektronik GmbH (APE) can provide an optical parametric oscillator (OPO), a tunable source for coherent anti-Stokes Raman scattering (CARS) or stimulated Raman scattering (SRS), and can offer a fully integrated system, whereas laser sources are incorporated in a single device that directly enables video-rate CARS or SRS imaging.
As important in Raman spectroscopy are light delivery and detection. Art photonics GmbH enables light delivery in different shapes and intensity profiles through a fiber, making it possible to integrate it into a clinical device (Figure 1c).
The main advantage of hyperspectral imaging is that it requires no prior knowledge of the sample, and postprocessing allows all available information from the data set to be mined. Hyperspectral images can be acquired in the visual and IR regions, but in either case a stable broadband light source is required. Achieving uniform illumination is also problematic.
Optical coherence tomography
Because optical coherence tomography (OCT) provides 2D and 3D images in living tissue and in real time, it has recently attracted much attention in the biophotonics scene.
Small aperture, poor lateral resolution, and low penetration depth are technical limitations of OCT. Nevertheless, commercial OCT systems are finding successful applications in ophthalmology, cardiovascular medicine, dentistry, and dermatology, as well as in other areas such as wafer inspection. To enable further development and propagation of this technique in the clinical environment, Fondazione CIFE offers an external cavity tunable laser for swept laser sources.
Swept laser sources enable design flexibility, high coherence, and high yield. At the same time, because of OCT’s very small footprint, portability can be targeted using a micro-optic assembly. A key enabler in facilitating the development of OCT systems for hand-held applications is photonic integrated circuit (PIC) technology. The lack of services for the final packaging of systems based on PICs can impede the commercialization of these devices. However, PIXAPP, the European pilot line for packaging and assembly of PIC-based products, offers a complete service with all of the required processes, including optical coupling, thermal stabilization, and electronic integration — all focusing on standards and automation.
Photoacoustic imaging
Thanks to recent technical progress, another technique gaining ground in biophotonics is photoacoustic imaging (Figure 2). In photoacoustic imaging, nonionizing laser pulses penetrate deep into biological tissues, where some of the energy is absorbed and converted into heat, which leads to ultrasonic emission. The magnitude of this ultrasonic emission reveals physiologically specific optical absorption contrast, enabling translation of the signal into 2D or 3D images of the targeted areas. When compared to conventional ultrasound, photoacoustic imaging therefore has the unique ability to visualize molecular changes deeper within living tissue, while retaining spatial resolution.
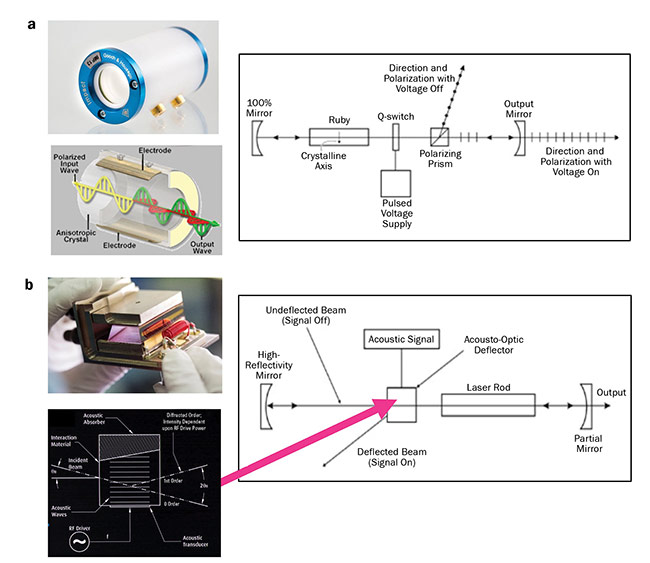
Figure 2. High-energy pulse generation in electro-optics (a) and acousto-optics (b). Also shown are examples of the chambers and
schematics of the pulse-generation mechanism. RF: radio frequency. Courtesy of Gooch & Housego.
With photoacoustic imaging, the laser source provides pulsed signals at a unique wavelength, with microjoule energy being compact, fiber coupled, and robust at the same time. New lasers are being manufactured to fulfill these requirements, and these components are aiding the development of multispectral optoacoustic tomography (MSOT) systems.
MSOT combines the molecular specificity of optical imaging with the depth and spatiotemporal resolution of ultrasound. Tissue is illuminated with laser pulses at multiple wavelengths and the induced ultrasound pressure wave is detected. Spectral unmixing allows analysis of individual absorbers. The resolution measures up to 80 µm, while the imaging depth measures several centimeters. MSOT allows whole-body imaging and has already been clinically tested.
Another development in laser technology is raster-scanning optoacoustic mesoscopy (RSOM), which utilizes laser excitation and high-frequency acoustic detection. In contrast to MSOT, RSOM uses a raster-scanning approach with a single-element ultrasound detector, thereby providing intrinsic optical tissue contrast with 10- to 20-µm resolution at several millimeters depth. Clinically, RSOM can be used to detect diseases in which irregular accumulation of hemoglobin or melanin is involved at mesoscopic scales (Figure 3).
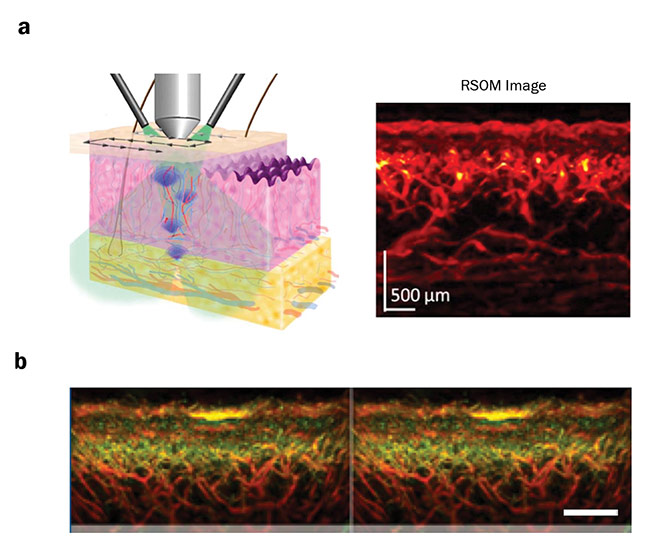
Figure 3. A raster-scanning optoacoustic mesoscopy (RSOM) scan of skin with the resulting image (a). The different layers of the skin can be clearly distinguished. Benign nevus on human forearm, shown in yellow; smaller vessels are shown in green and bigger vessel in red (b).
Courtesy of iTheraMedical.
Laser surgery
In addition to enabling noninvasive imaging of tissue, lasers have a wide range of applications in surgery, such as in endarterectomy (with an argon laser), in healing and reshaping the cornea, and in laser-assisted dentistry, and cosmetic and skin treatments. There is even a carbon dioxide laser scalpel.
During an EPIC meeting in December 2018 in Copenhagen, Denmark, Microrelleus SL presented a new application for laser-assisted surgeries in the form of laser-based implant texturing (Figure 4). Functional texturing can offer new properties to an implant, such as reducing its friction or making it superhydrophobic or superhydrophilic, self-cleaning, aseptic, and self-lubricating. An appropriate combination of functional texturing improves osseointegration (bone attachment to or growth into the device). Among the most important advantages of laser texturing are manufacturing benefits, risk and cost reduction, and design benefits.
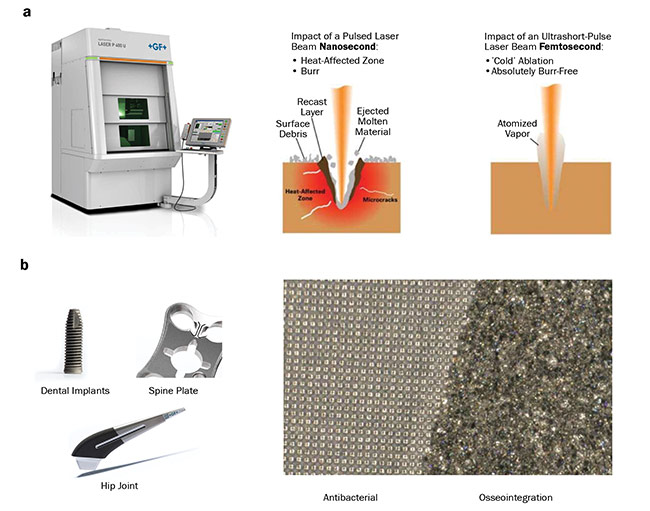
Figure 4. Laser-based texturing machinery (a, left) and a comparison of the impact of the laser beam of different lengths on the
material surface (a, right). Possible applications of laser texturing for clinical use (b). Courtesy of Microrelleus SL.
Future prospects
New developments in laser technologies will make it possible for numerous exciting applications to be incorporated into clinical practice. Among them, Raman and hyperspectral imaging will allow direct evaluation of tumor margins during surgery. Brought to common practice, OCT and photoacoustic imaging systems will take the diagnosis of skin diseases to a new level of precision.
Further improvements in laser technology will also stimulate the advancement of cosmetic surgeries and ophthalmological treatments. These and many other applications will become available as a result of the joint efforts of the photonics community.
Meet the authors
Jose Pozo, Ph.D., is director of technology and innovation at the European Photonics Industry Consortium (EPIC). He has a 15-year background in photonics technology and market knowledge and a large network within the industrial and academic photonics landscape. He is a member of the board of the IEEE Photonics Society Benelux. He has a doctorate in electrical engineering from the University of Bristol in England and bachelor’s and master’s degrees in telecom engineering.
Ana Belén González Guerrero, Ph.D., is a project leader at EPIC. Her expertise lies in the development of systems based on integrated photonic circuits, packaging and assembly, and the investigation of applications such as chemical/biological sensing and Datacom. In addition, she has been involved in technology transfer and business development processes. She has a doctorate from the Catalan Institute of Nanoscience and Nanotechnology and a bachelor’s degree in chemistry from the Autonomous University of Barcelona.
Acknowledgments
This article offers a summary of technical advancements in lasers and imaging presented during the EPIC meeting and workshop on medical lasers and biophotonics, held in December 2018 in Copenhagen, Denmark.
The authors would like to thank the following attendees for their active participation at the presentation and in follow-up discussions, with special thanks to the EPIC members (in italics): Antoni van Leeuwenhoek Hospital, Angewandte Physik & Elektronik (APE), Arden Photonics Ltd., art photonics GmbH, Asphericon GmbH, Astrum, Chroma Technology Corp., Coherent, CSEM, Delta Optical Thin Film, Eclipse Optics, EKSPLA, ETH Zurich, FISBA, Focuz, Fondazione CIFE, Gooch & Housego, HÜBNER Photonics, iThera Medical, Laservision, Leibniz Institute of Photonic Technology, Leiden University Medical Center, LEONI Fiber Optics Inc., Light Conversion, LUMIBIRD, Lumics GmbH, Lund University, Microrelleus SL, monocrom, nanoplus, NKT Photonics A/S, NorthLab Photonics AB, NYFORS Teknologi AB, OAL Innovations, OCTLIGHT, OFS, Oplatek Group, PhotoSana, PicoLAS GmbH, Politecnico di Torino (POLITO), Rockley Photonics, Santec Europe, SUSS MicroOptics, TOPTICA Photonics, The Tyndall National Institute (TNI), Univet, VALO Innovations, X-Celeprint, and Yelo Ltd.