Low-level laser instruments continue to evolve and be used to explore new therapeutic applications, from treating COVID-19 to mitigating pain.
JAMES SCHLETT, CONTRIBUTING EDITOR
One month after the World Health Organization declared the coronavirus a pandemic in March 2020, Brazilian researchers at Hospital Tacchini in Rio Grande do Sul invited Multi Radiance Medical to collaboratively explore a novel idea. The researchers wanted the Ohio-based developer of therapeutic lasers to be part of a clinical trial to explore the effects of photobiomodulation therapy (PBMT) on the respiratory muscles of COVID-19 patients.
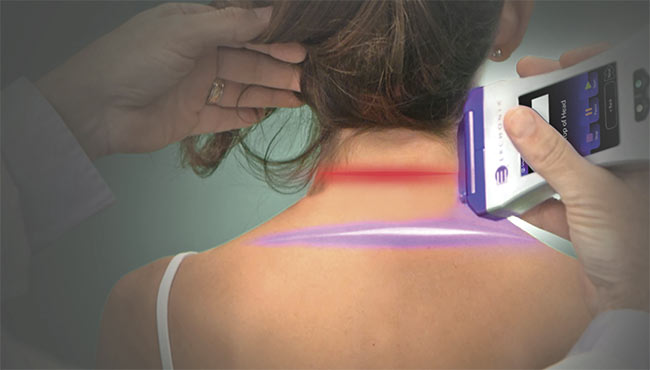
While low-level laser therapies have generally relied on thermal effects in tissue as the basis for treatment, nonthermal technologies are beginning to gain traction. The first nonthermal laser therapy to receive FDA clearance for treating pain indication with a violet laser only emerged in 2019. The clearance allowed the use of 405-nm laser diode light to provide temporary relief of musculoskeletal-based chronic neck and shoulder pain. Courtesy of Erchonia.
Multi Radiance develops so-called superpulsed laser technology based on diode lasers. The instruments are capable of packing up to 50,000 mW of peak power into optical pulses that last billionths of a second. The result, the company claims, is that its sources can deliver a higher concentration of photons deeper into targeted tissue without any risk of overheating.
Multi Radiance had previously studied its technology’s ameliorative effects on patients with chronic obstructive pulmonary disease, with a focus on reducing the symptoms of breathing difficulty (dyspnea). However, the team at the hospital,
led by Fabiano Francio, the coordinator of physiotherapy service, wanted to see whether combining photobiomodulation
therapy with a static magnetic field (PBMT-sMF) could preserve respiratory muscle and control the inflammation process that stems from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of COVID-19. The results of the clinical trial convinced Multi Radiance to pursue 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its pulsed laser technology for the treatment of COVID-19 patients.
The collaboration between Multi Radiance and Francio’s team illustrates the emerging therapeutic opportunities for low-level lasers used in PBMT, especially nonthermal methods. Thermal low-level laser therapy (LLLT) devices, however, remain a driving force and continue to be increasingly popular, especially in the wake of the FDA’s 2017 determination that certain IR laser devices that result in topical heating are exempt from the 510(k) premarket notification requirements.
“The area of pulmonology has not likely heard of this technology, and it will have a great deal to overcome,” said Douglas Johnson, Multi Radiance’s senior vice president of clinical and scientific affairs. “During the pandemic, everything has been put up as a possible cure or treatment for COVID. There needs to be good science during these times. We focused our research on an area where there is little to no other forms of care — the end stages of mechanical ventilation. This is where the impact can be best seen as helping to improve how we care for those critically ill and with no other options available.”
Clinical trials
Multi Radiance’s three-month clinical trial started in May 2020 and focused on adult COVID-19 patients who had been put on a ventilator in Hospital Tacchini’s intensive care unit (ICU) due to respiratory failure. The patients were treated with a prototype of Multi Radiance’s MR5 ACTIV PRO LaserShower, according to a paper on the study published this past July in the Journal of Inflammation1. The multiwavelength device incorporates three diode arrays: one comprising four 905-nm laser diodes, a second comprising eight LEDs emitting at 633 nm, and a third comprising eight LEDs emitting at 850 nm.
Johnson said the researchers chose the MR5 because the size of its aperture minimized the time that the physical therapy team would be exposed to ventilated patients.
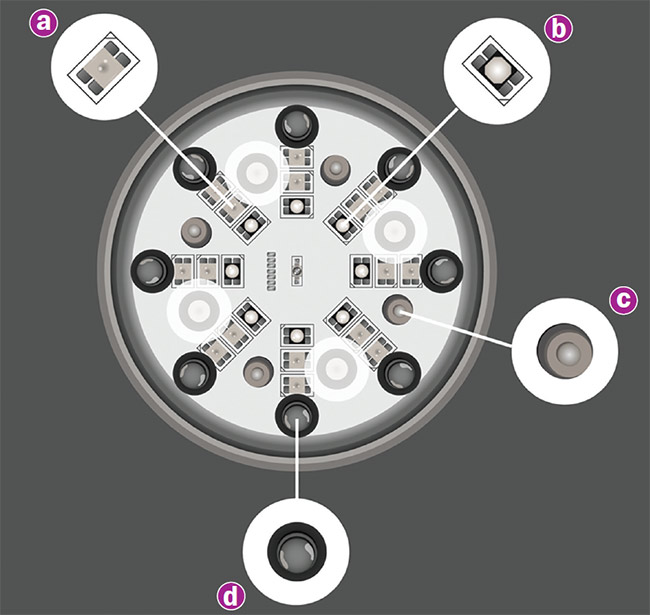
A recent clinical trial determined that a prototype combining red (a) and infrared LEDs (b), a superpulsed diode laser (c), and a magnetic field (d) did not show statistical significance in reducing the intensive care unit stays of severe COVID-19 patients. But the instrument did produce several positive results, including increased diaphragm thickness, a higher ratio of arterial oxygen partial pressure to fractional inspired oxygen (PO2/FiO2), and an elevated lymphocyte count. Courtesy of Multi Radiance Medical.
“We utilize a multilight approach, focusing on the value and benefits that each type of light-emitting diode offers,” he said. “Superpulsing by nature has a better depth of penetration, while LEDs help to cover larger areas with fewer treatment sites. Our research in over 30 trials finds that the combination of the light sources is beneficial to providing a synergistic and therapeutic effect not seen by either technology individually.”
The device delivered PBMT-sMF at several sites on each patient’s lower thorax/upper abdominal cavity and neck area. Although the treatment did not show statistical significance in reducing the ICU stays of severe COVID-19 patients who had been put on a ventilator, it did show several positive results: increased diaphragm thickness, a higher ratio of arterial oxygen partial pressure to fractional inspired oxygen (PO2/FiO2), and an elevated lymphocyte count. There were also decreases in FiO2, C-reactive protein levels, and hemoglobin count.
Even though the FDA classifies the MR5 as a thermal-based therapeutic product, Johnson said the effects seen in the clinical trial were not based on thermal changes from IR energy. The device settings applied in the study did not deliver sufficient energy to significantly increase skin temperature. “These [treatments] were done [for] less than one minute per location. The MR5 is not listed for sale for improving diaphragm dysfunction currently,” he said. “In theory, a nonthermal laser should have the same effect.”

Multi Radiance Medical’s MR4 was the company’s
first system to receive FDA clearance as a
nonthermal laser device for pain therapy or related indications. Courtesy of Multi Radiance Medical.
In fact, this past summer, Toronto-based Vielight entered the last stage of a clinical trial involving 280 moderate to severe COVID-19 patients who received PBMT with its Vielight RX Plus. The study combines the delivery of LED-based 633-nm red light into the nose and LED-based 810-nm NIR light to the thymus gland and lungs. While LEDs were used in this study, Vielight offers a similar intranasal delivery device that uses a 655-nm red laser.
The company’s founder and CEO, Lew Lim, said that 633- and 810-nm wavelengths are within the traditional spectrum of photobiomodulation devices, and Vielight offers its 655-nm laser because the laser’s wavelength has demonstrated good efficacy. He said that if the 655-nm laser had been used in the recent clinical trial instead of LEDs, the results might have been marginally different. Since the RX Plus device used in the study is intended for home use, LED light sources offered a higher degree of safety versus a laser-based instrument.

Vielight was recently involved in a clinical trial on the effectiveness of treating COVID-19 patients through the intranasal delivery of 633-nm red
light and the delivery of 810-nm IR light to the thymus gland and lungs. Courtesy of Vielight.
“In my opinion, photobiomodulation should avoid thermal effect for optimal effect, except for pain treatment, where some heat seems to help,” Lim said. “Biologically, heat stress decreases cell viability and proliferation while inducing apoptosis and necrosis. I would say heat compromises the effect of PBM.”
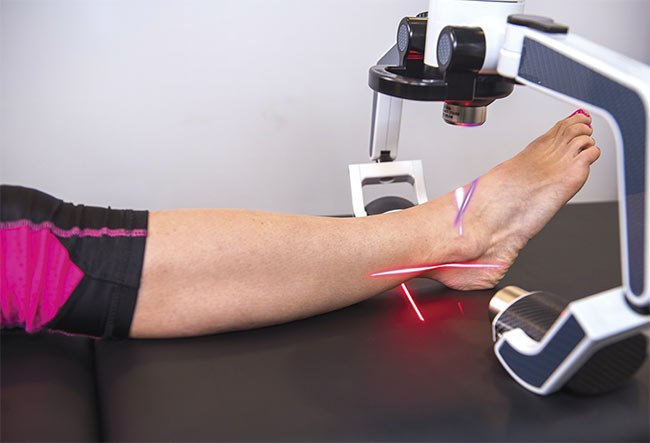
In addition to recently receiving FDA 510(k) clearance for treating pain indication using a violet laser, Erchonia is exploring using green light for pain and is sponsoring an active clinical trial using its 635-nm red laser device on diabetic peripheral neuropathy pain. Courtesy of Erchonia.
While Vielight’s technologies are available in the U.S. as low-risk general devices, Lim said the company is conducting several clinical trials and taking steps to procure regulatory clearances from the FDA and international agencies. As of August, the National Institutes of Health listed four Vielight-related clinical trials that are either active or recruiting for interventions of COVID-19, Alzheimer’s disease, and traumatic brain injury.
Thermal vs. nonthermal
In 2018, Multi Radiance received FDA 510(k) clearance for the MR4 laser, its first nonthermal laser device for pain therapy and related indications. The following year, Erchonia — which helped pioneer nonthermal laser therapies — received the first-ever 510(k) clearance for treating pain indication with a violet laser. The clearance allowed the Florida company to market its diode-based 405-nm EVRL laser for the temporary relief of musculoskeletal-based chronic neck and shoulder pain.
With the FDA previously only approving the use of violet light for conditions such as acne, Erchonia’s 510(k) for the EVRL pushed the boundaries of LLLT when used to treat pain. The roots of Erchonia’s technology trace back to 2002, when the company launched a 635-nm red laser that was the first to ever receive 510(k) clearance under FDA’s product classification of a nonthermal laser instrument for adjunctive use in pain therapy. Since then, the FDA has cleared 48 other systems under that classification, according to the agency’s medical device database.
Erchonia’s 2019 FDA clearance was based on an earlier clinical trial for treating neck and shoulder pain using its red laser technology, except instead of the 635-nm red laser, the company chose to apply its 405-nm violet laser. One challenge in the 2019 trial was that treatment of the neck and shoulder required delivery of laser energy over a large area. To achieve this, the company redesigned its optics to enable a line-generated laser system, making the laser more effective from a clinical perspective.
“The thought in the research community is that you need longer infrared lasers to penetrate for pain conditions,” said Steven Shanks, Erchonia’s president. “Erchonia has never bought into that theory, and instead of using longer wavelengths, we used shorter more energetic wavelengths. We believe the many FDA market clearances based on our research disproves that infrared wavelengths are more effective, and the myth about the depth of penetration of light into tissue is irrelevant.”
LLLT range
LLLT is generally viewed as most effective when it leverages visible or IR light using lasers emitting between 390 and 600 nm to treat superficial tissue and between 600 and 1100 nm to treat deeper tissue2. Erchonia’s instruments favor the lower end of the visible range. Shanks draws the line just past 700 nm. “Once you cross over 700 nm, this is considered infrared radiation and works through a different mechanism of action than visible light,” he said. “Infrared works through the molecular vibration (photophysical) that causes heating. Visible radiation is nonthermal and works through absorption of light causing electron transitions (photochemistry).”
Based on research that Erchonia conducted with the University of Illinois, the 405-nm wavelength was found to be optimal for treating fibrous tissue, Shanks said. The red 635-nm wavelength stimulated blood flow and mitochondrial activity, and increased adenosine triphosphate, which is critical for cellular energy storage and RNA synthesis.
Toronto-based BIOFLEX Laser Therapy, part of Meditech International, straddles this 700-nm threshold with therapeutic laser systems that use both the 660- and 880-nm wavelengths. These wavelengths are the most effective for
targeting the main photoacceptor in LLLT, mainly cytochrome c oxidase, the fourth and final step in the mitochondrial electron transport chain. The BIOFLEX lasers emit almost 200 mW of energy, which is important because power above 500 mW will cause thermal effects that inhibit the desired photobiomodulation effects of injured and diseased tissues, according to David Kunashko, Meditech’s director of education and training, and Ronaldo Santiago, Meditech’s director
of research.
Kunashko said LLLT provides fast relief from pain by directly inhibiting the sensory receptors for painful stimuli, which, in turn, inhibits second-order neurons. “Red wavelengths are thought
to be very effective for this,” he said. “But infrared is also recommended for deeper pain nerve fibers. Shorter wavelengths like blue and violet are not considered to be effective for direct pain relief.” As of August, BIOFLEX was collaborating in
a clinical trial that was in the recruitment stage for a study on the effects of red
and IR lasers on reducing pain experienced by women with provoked vestibulodynia.
“There are penetration limits with shorter wavelengths like blue and violet, but these are quite effective for dermatological disorders and wound care, and there is a trend to include these wavelengths when treating these types of conditions,” Kunashko said. “Longer NIR wavelengths that target water and certain ion channels are definitely the next frontier.”
References
1. T. De Marchi et al. (July 2021). Effects of photobiomodulation therapy combined with static magnetic field in severe COVID-19 patients requiring intubation: a pragmatic randomized placebo-controlled trial. J Inflammation Res, Vol. 14, pp. 3569-3585.
2. A. Pinar et al. (March 2013). Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutaneous Med Surg, Vol. 32, No. 1, pp. 41-52.